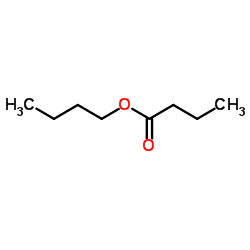
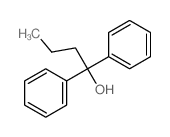
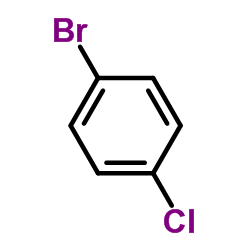
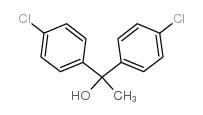
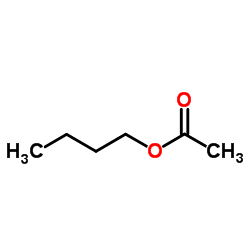
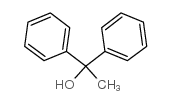
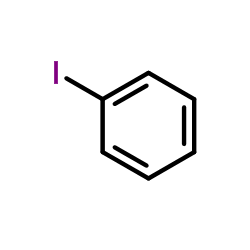
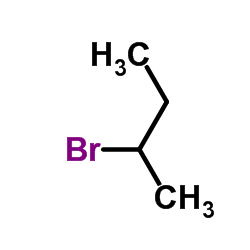
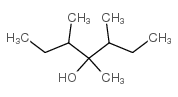
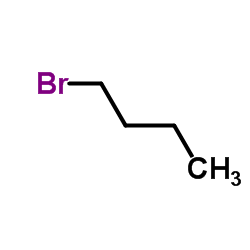
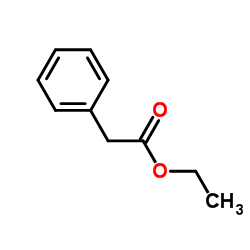
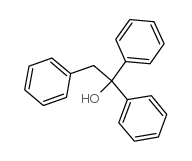
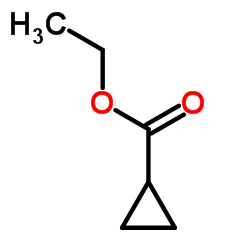
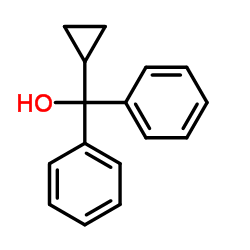
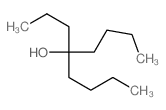
|
~87% |
|
~86% |
|
~89% |
|
~82% |
|
~93% |
|
~91% |
|
~51% |
|
~74% |
|
~83% |
|
~84% |
|
~58% |