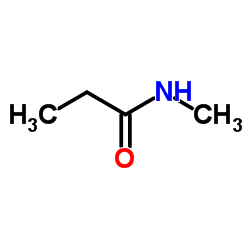
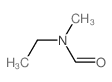
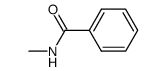
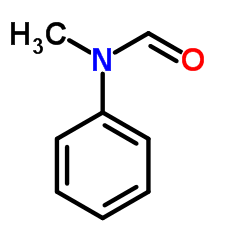
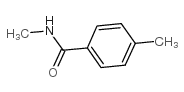
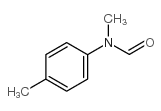
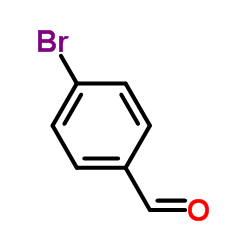
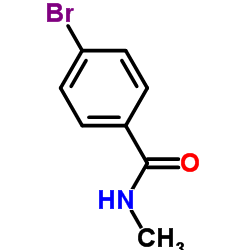
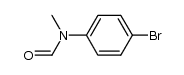
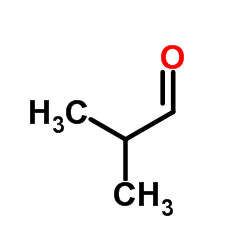
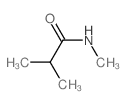
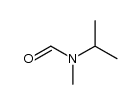
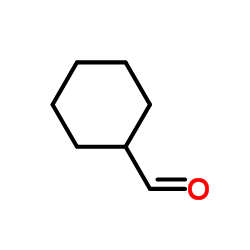
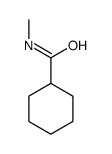
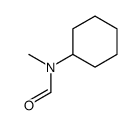
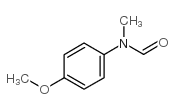
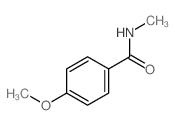
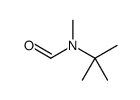
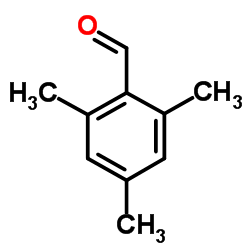
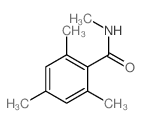
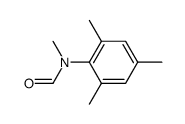
|
~8% |
|
~13% |
|
~9% |
|
~16% |
|
~67% |
|
~59% |
|
~66% |
|
~% |
|
~76% |
|
~34% |
|
~36% |