Lecture: fotemustine in brain tumors.
A Silvani, P Gaviani, E Lamperti, A Botturi, D Ferrari, G Simonetti, A Salmaggi
Index: Neurol. Sci. 32 Suppl 2 , S255-7, (2011)
Full Text: HTML
Abstract
Fotemustine (FTMS) is a third-generation nitrosourea, in preclinical studies, FTMS compared favorably with carmustine (BCNU) and lomustine (CCNU) against several human tumor cell lines. In conventional schedule, FTMS is administered at a dose of 100 mg/sqm/week for three consecutive weeks as induction (I) treatment, followed by 100 mg/sqm every three weeks, after a 5-week rest, as maintenance (M). Several Italian groups reported the results using FTMS in malignant glioma patients recurring after temozolomide standard treatment. In these papers, the 6-progression free survival are ranging from 20 to 52%. With the schedule (I + M) myelosuppression is observed in more than 30% of patients, and thrombocytopenia and leukopenia are more frequent and significant in Temozolomide pretreated patients. On the bases of the hematological toxicities several authors experimented new schedules of FTMS administrated at low doses. Recently, some authors reported the interesting results of a multicenter study on recurrent glioblastoma multiforme patients combining FTMS with new antiangiogentic agent bevacizumab.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
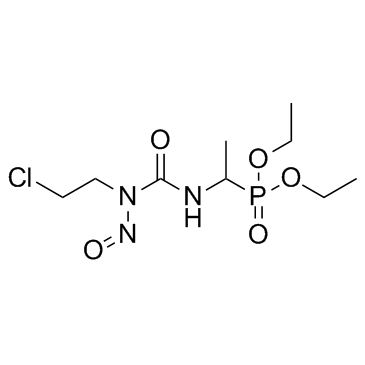 |
Fotemustine
CAS:92118-27-9 |
C9H19ClN3O5P |
|
Multiple bone metastases from glioblastoma multiforme withou...
2013-01-01 [Tumori 99(5) , e237-40, (2013)] |
|
Phase III randomized study of fotemustine and dacarbazine ve...
2013-01-01 [J. Transl. Med. 11 , 38, (2013)] |
|
Good clinical activity and favorable toxicity profile of onc...
2013-02-01 [Am. J. Hematol. 88(2) , 102-6, (2013)] |
|
High activity of sequential low dose chemo-modulating Temozo...
2010-01-01 [J. Transl. Med. 8 , 115, (2010)] |
|
Fotemustine in the treatment of brain primary tumors and met...
1994-01-01 [Cancer Invest. 12(4) , 414-20, (1994)] |