Fotemustine in the treatment of brain primary tumors and metastases.
D Khayat, B Giroux, J Berille, V Cour, B Gerard, M Sarkany, P Bertrand, J P Bizzari
Index: Cancer Invest. 12(4) , 414-20, (1994)
Full Text: HTML
Abstract
Fotemustine is a new chloroethylnitrosourea characterized by the grafting of a phosphonoalanine group onto a nitrosourea radical. Clinical studies using fotemustine have been conducted in malignant glioma, brain metastasis of non-small cell lung cancer, and disseminated malignant melanoma. In recurrent malignant glioma, fotemustine has been used as a single agent: assessed by computed tomography scan, after 8 weeks, the objective response rate was 26.3% among 38 evaluable patients. Median duration of response was 33 weeks. The main toxicity was hematological (thrombocytopenia and leucopenia). A trial with high-dose fotemustine and autologous bone marrow rescue in newly diagnosed glioma was conducted in 26 patients, and 6 showed a partial response. The median overall survival was approximately 11 months. Myelosuppression was noted in all patients except 1, and other toxicity reported was central nervous system toxicity and epigastric pain. Combined with radiotherapy in 55 patients, a 29% response rate was observed, and this combination was well tolerated and easily manageable on an outpatient basis. Finally, fotemustine has been used intraarterially, with 10 objective responses observed among 26 evaluable patients. In brain metastases of non-small cell lung cancer, fotemustine proved to be active with a response rate of 16.7%. Combined with cisplatinum, fotemustine is still under study, but preliminary results are promising. In cerebral metastases of disseminated malignant melanoma, fotemustine has been evaluated in a total of 140 patients in the various studies: median response rate is 24.3%, ranging from 8.3% to 60.0%. Fotemustine appears to be a good candidate in the treatment of primary brain tumors and metastases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
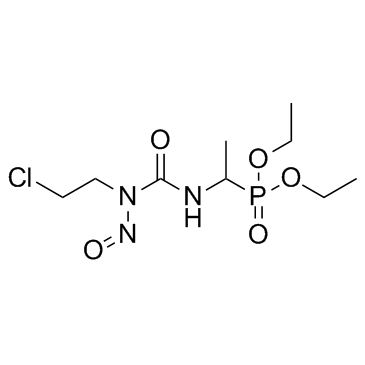 |
Fotemustine
CAS:92118-27-9 |
C9H19ClN3O5P |
|
Multiple bone metastases from glioblastoma multiforme withou...
2013-01-01 [Tumori 99(5) , e237-40, (2013)] |
|
Phase III randomized study of fotemustine and dacarbazine ve...
2013-01-01 [J. Transl. Med. 11 , 38, (2013)] |
|
Good clinical activity and favorable toxicity profile of onc...
2013-02-01 [Am. J. Hematol. 88(2) , 102-6, (2013)] |
|
High activity of sequential low dose chemo-modulating Temozo...
2010-01-01 [J. Transl. Med. 8 , 115, (2010)] |
|
Proton inactivation of melanoma cells enhanced by fotemustin...
2011-02-01 [Radiat. Prot. Dosimetry 143(2-4) , 503-7, (2011)] |