Twice-daily dosing of temozolomide in combination with fotemustine for the treatment of patients with refractory glioblastoma.
M Santoni, A Paccapelo, L Burattini, A Onofri, S Cascinu
Index: Anticancer Res. 32(3) , 1099-101, (2012)
Full Text: HTML
Abstract
Alkylating agents, such as temozolomide (TMZ) and fotemustine (FTM) are widely used in recurrent glioblastoma (GBM) regimes. Several strategies have been proposed to prevent resistance to these agents, by combining or sequencing them. We report the results of a pilot study of patients with refractory GBM receiving a regime of twice-daily dosing of temozolomide administered on day 1, (with an initial oral dose of 200 mg/m(2) and a second oral dose of 75 mg/m(2) 12 h later), followed by fotemustine in a single i.v. infusion at 75 mg/m(2) on day 2, repeated every four weeks. Enrolment was stopped at 15 patients due to lack of effectiveness of this schedule for patients with GBM. Toxicity was mild, with no grade 4 side effects reported. Results indicate that our temozolomide -FTM combined schedule is not effective, although well tolerated, in non responsive patients with GBM. Further strategies are required to improve the outcome of these patients.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
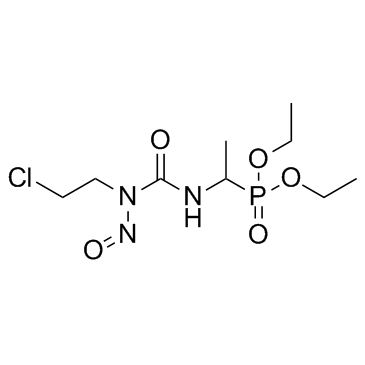 |
Fotemustine
CAS:92118-27-9 |
C9H19ClN3O5P |
|
Multiple bone metastases from glioblastoma multiforme withou...
2013-01-01 [Tumori 99(5) , e237-40, (2013)] |
|
Phase III randomized study of fotemustine and dacarbazine ve...
2013-01-01 [J. Transl. Med. 11 , 38, (2013)] |
|
Good clinical activity and favorable toxicity profile of onc...
2013-02-01 [Am. J. Hematol. 88(2) , 102-6, (2013)] |
|
High activity of sequential low dose chemo-modulating Temozo...
2010-01-01 [J. Transl. Med. 8 , 115, (2010)] |
|
Fotemustine in the treatment of brain primary tumors and met...
1994-01-01 [Cancer Invest. 12(4) , 414-20, (1994)] |