A thioamide substrate of carboxypeptidase A.
P A Bartlett, K L Spear, N E Jacobsen
Index: Biochemistry 21(7) , 1608-11, (1982)
Full Text: HTML
Abstract
Carbobenzoxythioglycyl-L-phenylalanine [CbzNHCH2C(==S)Phe, Z-Glys-Phe] was synthesized as thioamide analogue of Z-Gly-Phe, a known substrate of carboxypeptidase A (CPA). By use of a ninhydrin-based assay and Z-Gly-Gly-Phe as the substrate, Z-Glys-Phe was shown to be a weak competitive inhibitor of CPA (Ki = 1.4 mM). The L isomer (but not the D) of Z-Glys-Phe proved to be a substrate for CPA (Km = 1.1 mM and kcat = 5.3 s-1 at pH 7.5), binding with comparable affinity to, but hydrolyzing at 10% the rate of, the oxo analogue Z-Gly-Phe. The CPA-catalyzed hydrolysis of Z-Glys-Phe was shown to involve only C-N bond cleavage, to give carbobenzoxythioglycine and phenylalanine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
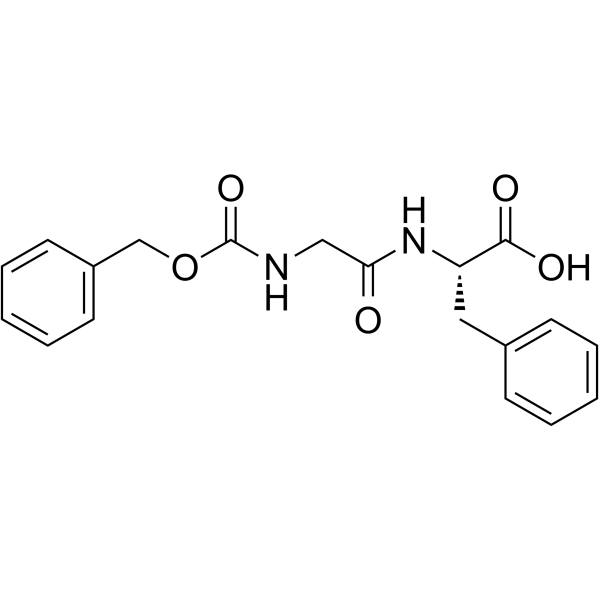 |
Z-Gly-Phe-OH
CAS:1170-76-9 |
C19H20N2O5 |
|
Virus replication inhibitory peptide inhibits the conversion...
1986-07-01 [Biosci. Rep. 6(7) , 647-53, (1986)] |
|
Synthesis and conformational study of a cyclic hexapeptide a...
1987-03-01 [J. Pept. Protein Res. 29 , 464, (1987)] |
|
Insulin receptor autophosphorylation and signaling is altere...
1995-02-14 [Biochemistry 34(6) , 1815-24, (1995)] |
|
Determination of pancreatic carboxypeptidase A in human bloo...
1983-11-30 [Clin. Chim. Acta 135(1) , 65-71, (1983)] |
|
Evidence suggesting a role for sperm metalloendoprotease act...
1988-11-01 [J. Exp. Zool. 248(2) , 213-21, (1988)] |