International Journal of Peptide and Protein Reseach
1987-03-01
Synthesis and conformational study of a cyclic hexapeptide analogue of somatostatin: cyclo(Phe-D-Trp-Lys-Thr-o-AMPA).
P Vander Elst, E van den Berg, H Pepermans, L vander Auwera, R Zeeuws, D Tourwe, G van Binst
Index: J. Pept. Protein Res. 29 , 464, (1987)
Full Text: HTML
Abstract
The active sequence Phe7-D-Trp8-Lys9-Thr10 of somatostatin has been cyclized through o-(aminomethyl)phenylacetic acid, a spacer molecule, designed to mimic a Gly-Gly dipeptide containing a cis-constrained peptide bond. The resulting analogue shows no GH-inhibition. A 2D n.m.r. study reveals conformations different from the proposed bio-active one and still sensitive to the medium (solvent).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
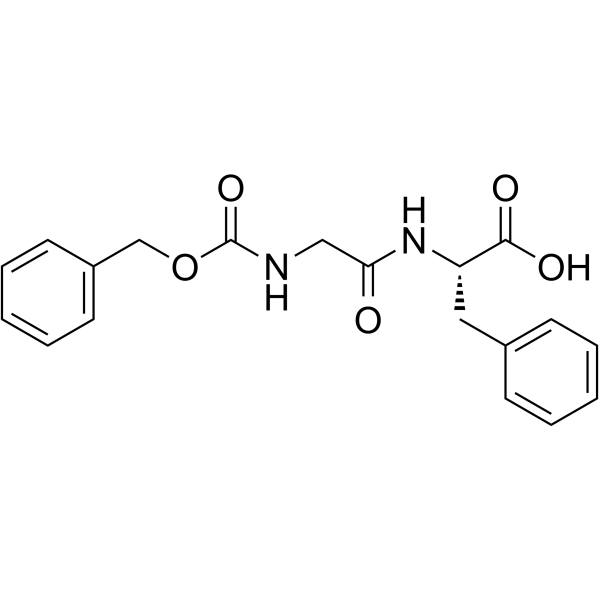 |
Z-Gly-Phe-OH
CAS:1170-76-9 |
C19H20N2O5 |
Related Articles:
More...
|
Virus replication inhibitory peptide inhibits the conversion...
1986-07-01 [Biosci. Rep. 6(7) , 647-53, (1986)] |
|
Insulin receptor autophosphorylation and signaling is altere...
1995-02-14 [Biochemistry 34(6) , 1815-24, (1995)] |
|
Determination of pancreatic carboxypeptidase A in human bloo...
1983-11-30 [Clin. Chim. Acta 135(1) , 65-71, (1983)] |
|
Evidence suggesting a role for sperm metalloendoprotease act...
1988-11-01 [J. Exp. Zool. 248(2) , 213-21, (1988)] |
|
Lysosomotropic agents. 4. Carbobenzoxyglycylphenylalanyl, a ...
1982-05-01 [J. Med. Chem. 25(5) , 539-44, (1982)] |