Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol.
G Kuznetsov, M A Brostrom, C O Brostrom
Index: J. Biol. Chem. 267(6) , 3932-9, (1992)
Full Text: HTML
Abstract
HepG2 cells were employed as model system to investigate potential relationships between early protein processing and Ca2+ storage by the endoplasmic reticulum. Ca2+ was required for glycoprotein processing and export by intact cells. The processing and export of alpha 1-antitrypsin and the secretion of complement factor 3, which are glycosylated proteins, were inhibited by the Ca2+ ionophore ionomycin whereas the export of albumin, a non-glycoprotein, was little affected. Ionomycin blocked processing of alpha 1-antitrypsin at the conversion from the high mannose to the complex glycosylated form without affecting ATP or GTP contents. Pre-existing inhibition of intracellular processing of alpha 1-antitrypsin by ionomycin was fully reversible upon removal of the ionophore with fatty acid-free bovine serum albumin. This reversal required Ca2+. After reversal the arrested form of alpha 1-antitrypsin was fully converted to the mature form and exported to the medium. Inhibitions of alpha 1-antitrypsin processing and complement factor 3 secretion by the metalloendoprotease antagonist Cbz-Gly-Phe-NH2 (where Cbz is benzyloxycarbonyl) were strongest at low extracellular Ca2+ but were reduced or prevented by high extracellular Ca2+. Processing and secretion of alpha 1-antitrypsin were reduced upon incubation in low Ca2+ medium. Exposure to dithiothreitol reduced albumin export while affecting alpha 1-antitrypsin export minimally. Suppression of amino acid incorporation into total cellular proteins of HepG2 cells accompanied inhibitions of protein processing by agents depleting sequestered Ca2+ stores or by dithiothreitol. Putative control of rates of translational initiation by the endoplasmic reticulum through linkage to rates of early protein processing is discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
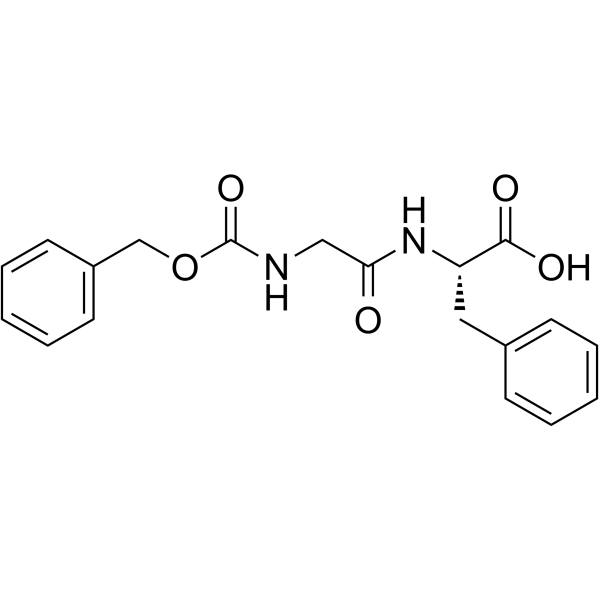 |
Z-Gly-Phe-OH
CAS:1170-76-9 |
C19H20N2O5 |
|
Virus replication inhibitory peptide inhibits the conversion...
1986-07-01 [Biosci. Rep. 6(7) , 647-53, (1986)] |
|
Synthesis and conformational study of a cyclic hexapeptide a...
1987-03-01 [J. Pept. Protein Res. 29 , 464, (1987)] |
|
Insulin receptor autophosphorylation and signaling is altere...
1995-02-14 [Biochemistry 34(6) , 1815-24, (1995)] |
|
Determination of pancreatic carboxypeptidase A in human bloo...
1983-11-30 [Clin. Chim. Acta 135(1) , 65-71, (1983)] |
|
Evidence suggesting a role for sperm metalloendoprotease act...
1988-11-01 [J. Exp. Zool. 248(2) , 213-21, (1988)] |