The development and application of a novel chromophoric substrate for investigation of the mechanism of yeast fatty acid synthase.
N Singh, S J Wakil, J K Stoops
Index: Biochem. Biophys. Res. Commun. 131(2) , 786-92, (1985)
Full Text: HTML
Abstract
The acetyl transacylase activity of the fatty acid synthase from yeast has been investigated using p-nitrophenylthiol acetate. The chromophoric nature of the nitrophenylthiol moiety affords a convenient spectrophotometric assay for the transacylase function as well as a means to investigate the kinetics and the mechanism of this process. A probable kinetic scheme for enzyme catalyzed transacetylation from p-nitrophenylthiol acetate to an acyl acceptor (CoA or N-acetylcysteamine) is proposed and the kinetic constants for acetylation of enzyme and for acetyl transfer to an acceptor were determined. It was also demonstrated that p-nitrophenylthiol acetate can replace acetyl-CoA as a substrate in fatty acid synthesis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
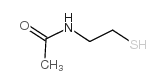 |
Acetamide,N-(2-mercaptoethyl)
CAS:1190-73-4 |
C4H9NOS |
|
Facile Synthesis and NO-Generating Property of 4H-[1,2,5]Oxa...
2001-01-01 [J. Org. Chem. 63(20) , 6947-6951, (1998)] |
|
[J. Chem. Soc. Perkin Trans. I , 2345, (1988)] |
|
[Tetrahedron Lett. 29 , 4305, (1988)] |
|
Isolation, properties, and regulation of a mitochondrial acy...
1979-06-10 [J. Biol. Chem. 254(11) , 4516-23, (1979)] |
|
Synthesis of the N-acetylcysteamine thioester of seco-proans...
2006-01-05 [Org. Lett. 8(1) , 135-8, (2006)] |