Facile Synthesis and NO-Generating Property of 4H-[1,2,5]Oxadiazolo[3,4-d]pyrimidine-5,7-dione 1-Oxides.
Magoichi Sako, Souichi Oda, Seiji Ohara, Kosaku Hirota, Yoshifumi Maki
Index: J. Org. Chem. 63(20) , 6947-6951, (1998)
Full Text: HTML
Abstract
4H-[1,2,5]Oxadiazolo[3,4-d]pyrimidine-5,7-dione 1-oxides (2) are conveniently prepared in high yields by the oxidative intramolecular cyclization of 6-amino-5-nitro-1H-pyrimidine-2,4-diones (1) employing iodosylbenzene diacetate as an oxidant in the presence of lithium hydride. The generation of nitric oxide (NO) and NO-related species from 2 occurs in the presence of thiols such as N-acetylcysteamine, cysteine, and glutathione under physiological conditions. The evidence for the NO generation derives from mechanistic interpretations for the reaction of 2 with thiols and other chemical observations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
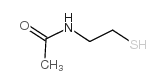 |
Acetamide,N-(2-mercaptoethyl)
CAS:1190-73-4 |
C4H9NOS |
|
[J. Chem. Soc. Perkin Trans. I , 2345, (1988)] |
|
[Tetrahedron Lett. 29 , 4305, (1988)] |
|
Isolation, properties, and regulation of a mitochondrial acy...
1979-06-10 [J. Biol. Chem. 254(11) , 4516-23, (1979)] |
|
Synthesis of the N-acetylcysteamine thioester of seco-proans...
2006-01-05 [Org. Lett. 8(1) , 135-8, (2006)] |
|
Reactions of acyl phosphates with carboxylate and thiol anio...
1982-09-25 [J. Biol. Chem. 257(18) , 10874-81, (1982)] |