| Structure | Name/CAS No. | Articles |
|---|---|---|
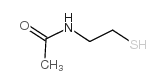 |
Acetamide,N-(2-mercaptoethyl)
CAS:1190-73-4 |
Thomas Frenzel, Marco Brünjes, Monika Quitschalle, Andreas Kirschning
Index: Org. Lett. 8(1) , 135-8, (2006)
Full Text: HTML
[structure: see text] The enantioselective total synthesis of the N-acetylcysteamine thioester of seco-proansamitocin, a key biosynthetic intermediate of the highly potent antitumor agent ansamitocin, is described, which twice utilizes the Nagao acetate aldol reaction, as well as an indium-mediated alkynylation of a benzyl bromide followed by carboalumination. The key step is a Heck reaction between two terminal alkenes for merging the two major fragments.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Acetamide,N-(2-mercaptoethyl)
CAS:1190-73-4 |
C4H9NOS |
|
Facile Synthesis and NO-Generating Property of 4H-[1,2,5]Oxa...
2001-01-01 [J. Org. Chem. 63(20) , 6947-6951, (1998)] |
|
[J. Chem. Soc. Perkin Trans. I , 2345, (1988)] |
|
[Tetrahedron Lett. 29 , 4305, (1988)] |
|
Isolation, properties, and regulation of a mitochondrial acy...
1979-06-10 [J. Biol. Chem. 254(11) , 4516-23, (1979)] |
|
Reactions of acyl phosphates with carboxylate and thiol anio...
1982-09-25 [J. Biol. Chem. 257(18) , 10874-81, (1982)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved