Full replacement of the function of the secondary electron acceptor phylloquinone(= vitamin K1) by non-quinone carbonyl compounds in green plant photosystem I photosynthetic reaction centers.
S Itoh, M Iwaki
Index: Biochemistry 30(22) , 5340-6, (1991)
Full Text: HTML
Abstract
One-carbonyl quinonoid compounds, fluorenone (fluoren-9-one), anthrone, and their derivatives are introduced into spinach photosystem (PS) I reaction centers in place of the intrinsic secondary electron acceptor phylloquinone (= vitamin K1). Anthrone and 2-nitrofluorenone fully mediated the electron-transfer reaction between the reduced primary electron acceptor chlorophyll A0- and the tertiary electron acceptor iron-sulfur centers. It is concluded that the PS I phylloquinone-binding site has a structure that enables various compounds with different molecular structures to function as the secondary acceptor and that the reactions of incorporated compounds are mainly determined by their redox properties rather than by their molecular structure. Carbonyl groups increase the binding affinity of the quinone/quinonoid compounds but do not seem to be essential to their function. The quinonoid compounds as well as quinones incorporated into the PS I phylloquinone-binding sites are estimated to function at redox potentials more negative than in organic solvents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
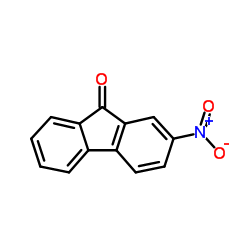 |
2-Nitro-9-fluorenone
CAS:3096-52-4 |
C13H7NO3 |
|
Laser desorption/ionization time-of-flight mass spectrometry...
1996-07-15 [Anal. Chem. 68(14) , 2319-24, (1996)] |
|
2-Nitrofluorene and related compounds: prevalence and biolog...
1988-09-01 [Mutat. Res. 196(2) , 177-209, (1988)] |
|
Voltammetric Determination of Genotoxic Nitro Derivatives of...
[Electroanalysis 22(17-18) , 2034-2042, (2010)] |
|
Isolation, identification and bacterial mutagenicity of 2-ni...
1986-02-01 [Mutat. Res. 173(2) , 105-9, (1986)] |
|
2-Nitrofluoren-9-one: a unique mutagen formed in the photo-o...
1986-03-01 [Carcinogenesis 7(3) , 499-502, (1986)] |