[Structure-release relationships of active polymeric drug combinations. 1. The influence of polymer structure on the diffusion of 3-methylpyrazole from monolithic systems].
B Schulz, P Pinther, M Hartmann
Index: Pharmazie 40(8) , 548-52, (1985)
Full Text: HTML
Abstract
The release of 3-methylpyrazole from monolithic polymer films into aqueous media has been studied. The diffusion of the active agent decreased with increasing of the content of acetate groups in reacetylated poly(vinyl alcohols) and with increasing of the ester lengths in copolymers of maleic esters, respectively. The release rate of active ingredient is dependent on the hydrophilicity of the polymer films. Contrary to the glass transition temperature the hydrophilicity of polymers expressed the interaction between polymer chains and water in a direct way. The hydrophilicity of copolymers of maleic esters correlated with the turbidimetric point, which can be determined by acidimetric titrations. The influences of interactions between polymers and the active agents on their diffusion in the polymers were also discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
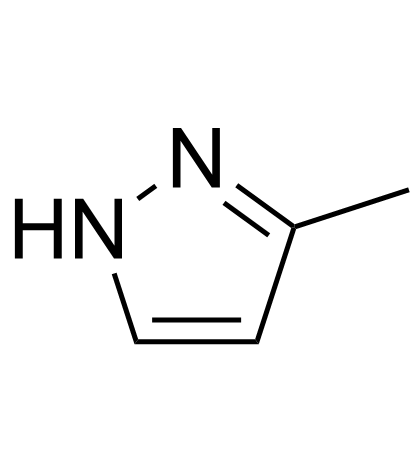 |
3-Methylpyrazole
CAS:1453-58-3 |
C4H6N2 |
|
[Toxicological evaluation of a new nitrification inhibitor a...
1986-06-01 [Gig. Sanit. (6) , 29-31, (1986)] |
|
Differential effects of 4-iodopyrazole and 3-methylpyrazole ...
1987-01-01 [J. Cancer Res. Clin. Oncol. 113(2) , 145-50, (1987)] |
|
Extremely long protection by pyrazole derivatives against ch...
1991-02-01 [J. Pharmacol. Exp. Ther. 256(2) , 592-8, (1991)] |
|
[Structure-release relationship of physical polymer/drug com...
1986-02-01 [Pharmazie 41(2) , 118-20, (1986)] |
|
Structure-release relationships of physically based polymer-...
1988-01-01 [Pharmazie 43(1) , 29-31, (1988)] |