GMDP: unusual physico-chemical and biological properties of the anomeriс forms.
Elena A Meshcheryakova, Konstantin S Mineev, Pavel E Volynski, Tatiana M Andronova, Vadim T Ivanov
Index: J. Pept. Sci. 21 , 717-22, (2015)
Full Text: HTML
Abstract
Disaccharide containing unit of peptidoglycan from bacterial cell wall, N-acetyl-d-glucosaminyl-N-acetylmuramyl-l-alanyl-d-glutaminamide (gluсosaminyl-muramyl-dipeptide) registered in Russia as an immunomodulatory drug, is shown to participate in slow equilibrium of α and β anomeric forms. Data of NMR spectra and molecular dynamics indicate that the α-anomer predominantly acquires a folded conformation stabilized by intramolecular hydrogen bond between the alanyl carbonyl and muramyl NH proton. The β-form displays a considerable fraction of extended, non-hydrogen bonded structures. In the standard immunoadjuvant test system, the α-form is practically inactive, and the activity of the equilibrium mixture with α : β = 68 : 32 ratio is due to the presence of β-anomer. Such unique α-β selectivity of biological action must be considered at the design of related immunoactive glycopeptides.Copyright © 2015 European Peptide Society and John Wiley & Sons, Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
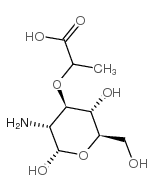 |
Muramic acid
CAS:1114-41-6 |
C9H17NO7 |
|
Differential response of human nasal and bronchial epithelia...
2015-01-01 [J. Toxicol. Environ. Health A 78 , 583-94, (2015)] |
|
Synergistic Effects Between Thioxanthones and Oxacillin Agai...
2015-08-01 [Microb. Drug Resist. 21 , 404-15, (2015)] |
|
The Staphylococcus aureus peptidoglycan protects mice agains...
2011-01-01 [PLoS ONE 6 , e28377, (2011)] |
|
Micromonospora maritima sp. nov., isolated from mangrove soi...
2013-02-01 [Int. J. Syst. Evol. Microbiol. , (2012)] |
|
High indoor microbial levels are associated with reduced Th1...
2012-01-01 [Int. Arch. Allergy Immunol. 159 , 194-203, (2012)] |