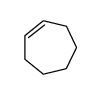
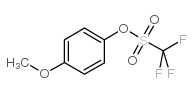
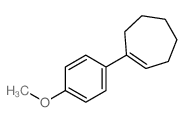
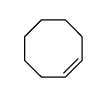
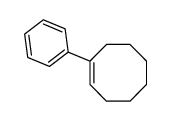
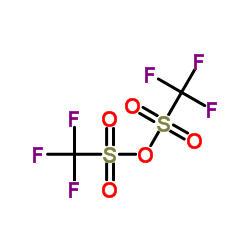
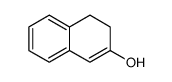
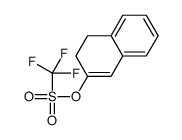
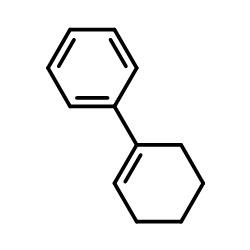
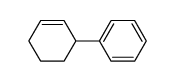
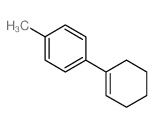
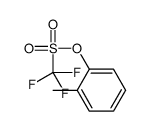
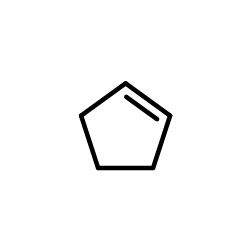
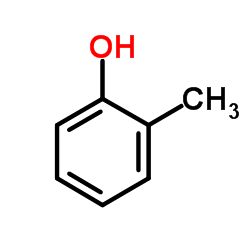
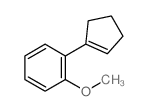
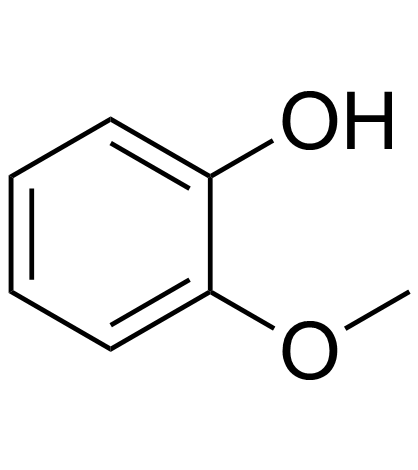
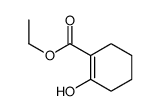
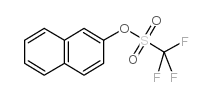
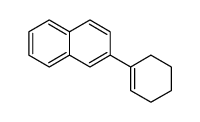
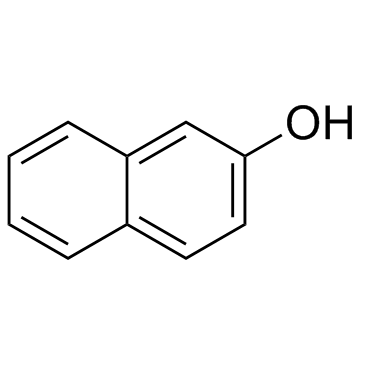
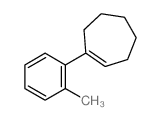
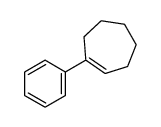
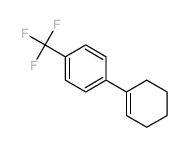
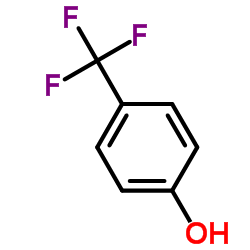
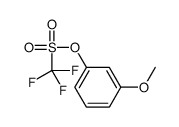
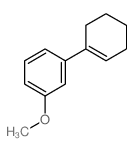
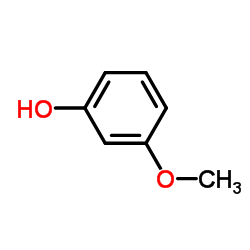
|
~89% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~%
Detail
|
|
~84% |
|
~% |
|
~10% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~87% |
|
~% |
|
~90% |
|
~92% |
|
~97% |
|
~% |
|
~89% |
|
~% |
|
~89% |
|
~% |
|
~86% |
|
~% |