Porcine pulmonary angiotensin I-converting enzyme--biochemical characterization and spatial arrangement of the N- and C-domains by three-dimensional electron microscopic reconstruction.
Hui-Ling Chen, Heinrich Lünsdorf, Hans-Jürgen Hecht, Hsin Tsai
Index: Micron 41(6) , 674-85, (2010)
Full Text: HTML
Abstract
The somatic angiotensin I-converting enzyme (sACE; peptidyl-dipeptidase A; EC 3.4.15.1) was isolated from pig lung and purified to homogeneity. The purified enzyme has a molecular mass of about 180 kDa. Upon proteolytic cleavage, two approximately 90 kDa fragments were obtained and identified by amino-terminal sequence analysis as the N- and C-domains of sACE. Both purified domains were shown to be catalytically active. A 2.3 nm resolution model of sACE was obtained by three-dimensional electron microscopic reconstruction of negatively stained sACE particles, based on atomic X-ray data fitting. Our model shows for the first time the relative orientation of the sACE catalytically active domains and their spatial distance.(c) 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
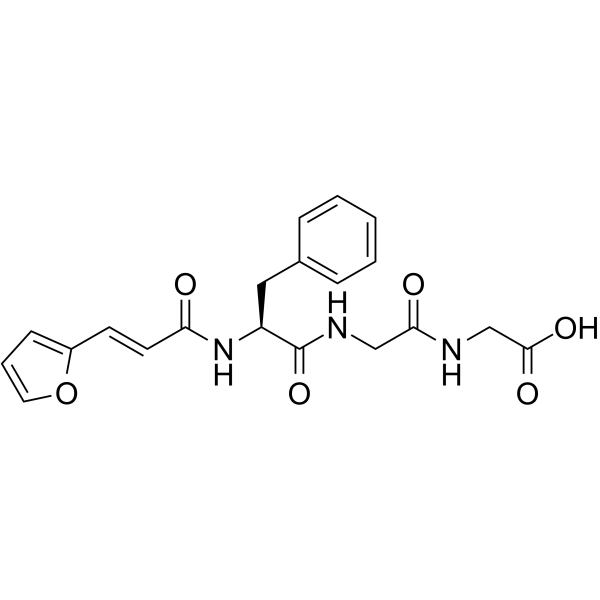 |
FA-Phe-Gly-Gly-OH
CAS:64967-39-1 |
C20H21N3O6 |
|
Effect of power ultrasound pretreatment on peptidic profiles...
2014-09-01 [J. Sci. Food Agric. 94(12) , 2420-8, (2014)] |
|
Assessment and optimization of kinetic methods for angiotens...
1993-02-01 [Clin. Chem. 39(2) , 312-6, (1993)] |
|
Evidence for the negative cooperativity of the two active si...
2003-08-28 [FEBS Lett. 550(1-3) , 84-8, (2003)] |
|
Angiotensin-converting enzyme determination in plasma during...
1991-08-01 [Clin. Chem. 37(8) , 1390-3, (1991)] |
|
Determination of enzyme (angiotensin convertase) inhibitors ...
2001-03-01 [J. Pharm. Biomed. Anal. 24(5-6) , 1151-6, (2001)] |