[Angiotensin-converting enzyme in an OAT-octane reversed micelle system: interaction with the matrix].
O A Kost, T A Ort, I I Nikol'skaia, S N Nametkin, A V Levashov
Index: Bioorg. Khim. 21(6) , 403-7, (1995)
Full Text: HTML
Abstract
Regulation of catalytic activity and intramolecular structure of the bovine lung angiotensin-converting enzyme was studied using reversed micelles in a sodium docusate-water-octane system, which model the enzyme's environment in vivo. The catalytic parameters of monomeric and dimeric forms of the enzyme in the reversed micellar system were evaluated. The catalytic activity of the angiotensin-converting enzyme extracted from bovine lung with Triton X-100 did not depend on the detergent concentration at a constant level of hydration. An artificially hydrophobized form of the angiotensin-converting enzyme was obtained by modifying the enzyme with stearic acid chloride. The modification leads to the dependence of the catalytic activity on the surfactant concentration, which provides evidence that the enzyme interacts with the micellar matrix. The modified enzyme showed a significant increase in catalytic activity in the reversed micellar system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
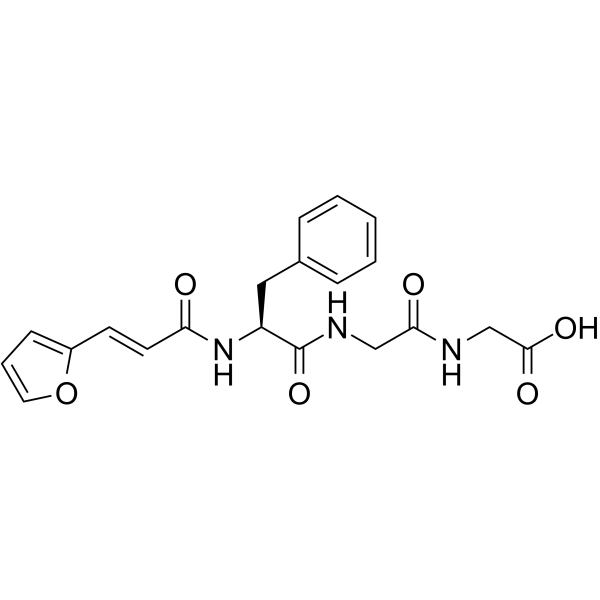 |
FA-Phe-Gly-Gly-OH
CAS:64967-39-1 |
C20H21N3O6 |
|
Effect of power ultrasound pretreatment on peptidic profiles...
2014-09-01 [J. Sci. Food Agric. 94(12) , 2420-8, (2014)] |
|
Assessment and optimization of kinetic methods for angiotens...
1993-02-01 [Clin. Chem. 39(2) , 312-6, (1993)] |
|
Evidence for the negative cooperativity of the two active si...
2003-08-28 [FEBS Lett. 550(1-3) , 84-8, (2003)] |
|
Angiotensin-converting enzyme determination in plasma during...
1991-08-01 [Clin. Chem. 37(8) , 1390-3, (1991)] |
|
Determination of enzyme (angiotensin convertase) inhibitors ...
2001-03-01 [J. Pharm. Biomed. Anal. 24(5-6) , 1151-6, (2001)] |