Colchicine glycorandomization influences cytotoxicity and mechanism of action.
Aqeel Ahmed, Noël R Peters, Megan K Fitzgerald, James A Watson, F Michael Hoffmann, Jon S Thorson
Index: J. Am. Chem. Soc. 128(44) , 14224-5, (2006)
Full Text: HTML
Abstract
The reaction of 70 unprotected, diversely functionalized free reducing sugars with methoxyamine-appended colchicine led to the production of a 58-member glycorandomized library. High-throughput cytotoxicity assays revealed glycosylation to modulate specificity and potency. Library members were also identified which, unlike the parent natural product (a destabilizer), stabilized in vitro tubulin polymerization in a manner similar to taxol. This study highlights a simple extension of neoglycorandomization toward amine-bearing scaffolds and the potential benefit of glycosylating nonglycosylated natural products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
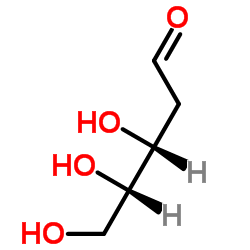 |
2-Deoxy-D-Ribose
CAS:18546-37-7 |
C5H10O4 |
|
The role of thymidine phosphorylase, an angiogenic enzyme, i...
2004-11-01 [Cancer Sci. 95(11) , 851-7, (2004)] |
|
Synthesis and biological activity of hydroxylated analogues ...
2013-04-05 [Carbohydr. Res. 370 , 46-66, (2013)] |
|
Warfarin glycosylation invokes a switch from anticoagulant t...
2011-08-01 [ChemMedChem 6(8) , 1347-50, (2011)] |
|
Inhibition of metastasis of tumor cells overexpressing thymi...
2004-03-01 [Cancer Res. 64(5) , 1794-801, (2004)] |
|
Molecular basis for the inhibition of hypoxia-induced apopto...
2002-03-08 [Biochem. Biophys. Res. Commun. 291(4) , 806-12, (2002)] |