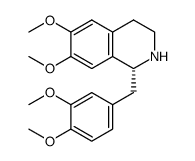
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~82% |
|
~% |
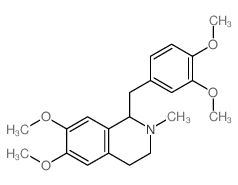
![1-[(3,4-Dimethoxyphenyl)methyl]-3,4-dihydro-6,7-dimethoxyisoquinoline Structure](https://image.chemsrc.com/caspic/235/6957-27-3.png)
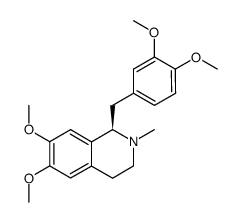
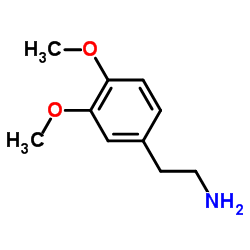
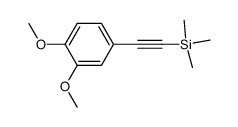
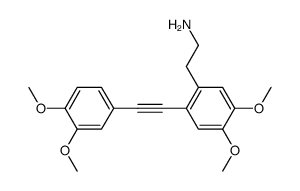
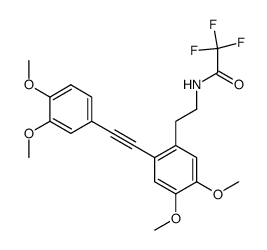
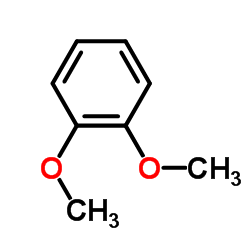
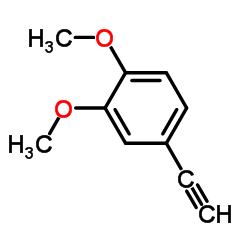
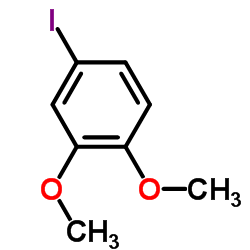
![N-[2-(3,4-dimethoxyphenyl)ethyl]-2,2,2-trifluoroacetamide Structure](https://image.chemsrc.com/caspic/283/13230-71-2.png)
![2,2,2-trifluoro-N-[2-(2-iodo-4,5-dimethoxyphenyl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/354/154138-43-9.png)