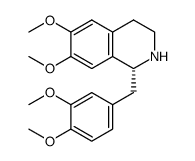
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~82% |
|
~% |

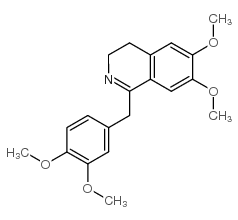
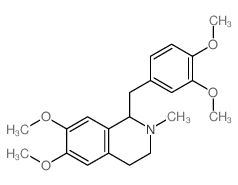
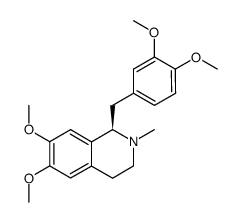
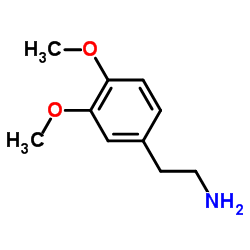
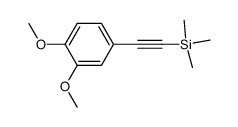
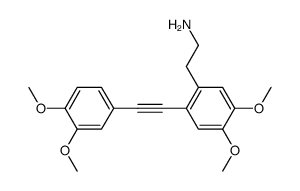
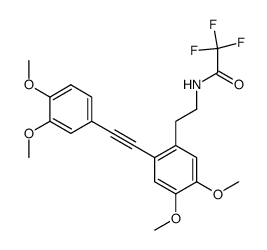
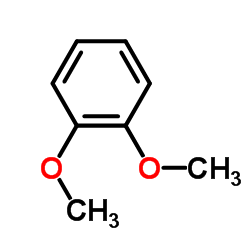
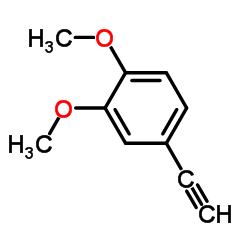
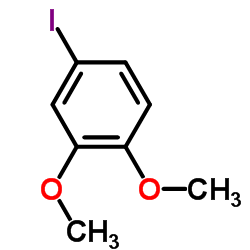
![N-[2-(3,4-dimethoxyphenyl)ethyl]-2,2,2-trifluoroacetamide结构式](https://image.chemsrc.com/caspic/283/13230-71-2.png)
![2,2,2-trifluoro-N-[2-(2-iodo-4,5-dimethoxyphenyl)ethyl]acetamide结构式](https://image.chemsrc.com/caspic/354/154138-43-9.png)