Expanding the repertoire of natural product-inspired ring pairs for molecular recognition of DNA.
Katy A Muzikar, Jordan L Meier, Daniel A Gubler, Jevgenij A Raskatov, Peter B Dervan
Index: Org. Lett. 13(20) , 5612-5, (2011)
Full Text: HTML
Abstract
A furan amino acid, inspired by the recently discovered proximicin natural products, was incorporated into the scaffold of a DNA-binding hairpin polyamide. While unpaired oligomers of 2,4-disubstituted furan amino acids show poor DNA-binding activity, furan (Fn) carboxamides paired with N-methylpyrrole (Py) and N-methylimidazole (Im) rings demonstrate excellent stabilization of duplex DNA as well as discrimination of noncognate sequences, consistent with function as a Py mimic according to the Py/Im polyamide pairing rules.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
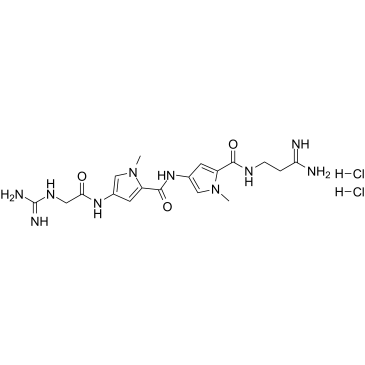 |
Netropsin dihydrochloride
CAS:18133-22-7 |
C18H28Cl2N10O3 |
|
Free energy calculations offer insights into the influence o...
2011-08-01 [J. Comput. Aided Mol. Des. 25(8) , 709-16, (2011)] |
|
Polyamide-scorpion cyclam lexitropsins selectively bind AT-r...
2011-01-01 [PLoS ONE 6(5) , e17446, (2011)] |
|
DNA site-specific N3-adenine methylation targeted to estroge...
2011-09-01 [Bioorg. Med. Chem. 19 , 5093-102, (2011)] |
|
Comparison of binding sites in DNA for berenil, netropsin an...
1987-09-01 [Eur. J. Biochem. 167 , 281, (1987)] |
|
[Estimation of activity of bis-netropsin derivatives based o...
2013-01-01 [Vopr. Virusol. 58(1) , 32-5, (2013)] |