| Structure | Name/CAS No. | Articles |
|---|---|---|
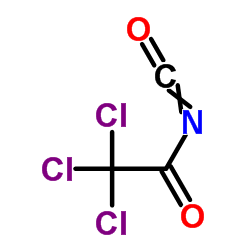 |
Trichloroacetyl isocyanate
CAS:3019-71-4 |
|
 |
N-(Trimethylsilyl)imidazole
CAS:18156-74-6 |
|
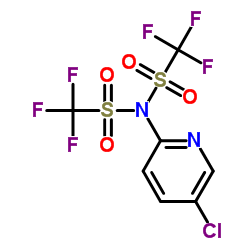 |
Comins' Reagent
CAS:145100-51-2 |