| Structure | Name/CAS No. | Articles |
|---|---|---|
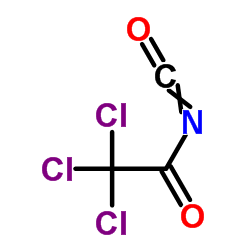 |
Trichloroacetyl isocyanate
CAS:3019-71-4 |
Tatsuya Shirahata, Jun-ichi Matsuo, Satoko Teruya, Nozomu Hirata, Taku Kurimoto, Nanao Akimoto, Toshiaki Sunazuka, Eisuke Kaji, Satoshi Omura
Index: Carbohydr. Res. 345(6) , 740-9, (2010)
Full Text: HTML
Efficient catalytic and stereoselective glycosylation was achieved by activating a glycosyl N-trichloroacetylcarbamate with a catalytic amount of Lewis acid in the presence of a glycosyl acceptor and 5A molecular sieves. Catalytic one-pot dehydrative glycosylation of a 1-hydroxy carbohydrate was achieved stereoselectively by reaction with trichloroacetyl isocyanate, followed by activation with a catalytic amount of activators.Copyright (c) 2010 Elsevier Ltd. All rights reserved.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Trichloroacetyl isocyanate
CAS:3019-71-4 |
C3Cl3NO2 |
|
Potential scans and potential energy distributions of normal...
2002-02-01 [J. Mol. Model. 8(2) , 44-9, (2002)] |
|
D.R. Taylor
[Can. J. Chem. 54 , 189, (1976)] |
|
M. Budesinsky et al.
[Collect. Czech. Chem. Commun. 45 , 2784, (1980)] |
|
H. Fujiwara, A.K. Bose
[Pract. Spectrosc. 3 , 329, (1980)] |
|
Determination of chain branching in epoxy resins by nuclear ...
1972-04-01 [Anal. Chem. 44 , 837, (1972)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved