| Structure | Name/CAS No. | Articles |
|---|---|---|
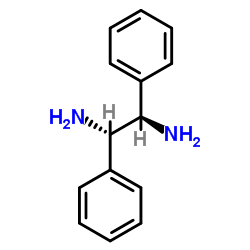 |
meso-1,2-Diphenylethylenediamine
CAS:951-87-1 |
|
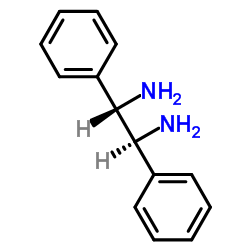 |
(1S,2S)-(-)-1,2-Diphenyl-1,2-ethanediamine
CAS:29841-69-8 |
|
 |
(1R,2R)-(+)-1,2-Diphenylethylenediamine
CAS:35132-20-8 |