| Structure | Name/CAS No. | Articles |
|---|---|---|
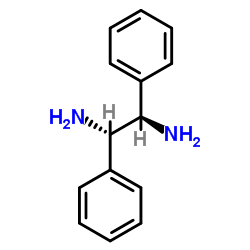 |
meso-1,2-Diphenylethylenediamine
CAS:951-87-1 |
Koichi Kodama, Ayaka Kanno, Eriko Sekine, Takuji Hirose
Index: Org. Biomol. Chem. 10(9) , 1877-82, (2012)
Full Text: HTML
A supramolecular chiral host consisting of N-(2-naphthoyl)-L-aspartic acid (L-1) and meso-1,2-diphenylethylenediamine (2) is effective in enantioseparation of 1-arylethanols (up to 96% ee with 100% inclusion ratio). Here we report three different methods to prepare the inclusion crystals and discuss the chiral recognition mechanism on the basis of X-ray crystallography results.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
meso-1,2-Diphenylethylenediamine
CAS:951-87-1 |
C14H16N2 |
|
Synthesis and characterisation of nickel Schiff base complex...
2015-02-21 [Dalton Trans. 44(7) , 3136-50, (2015)] |
|
Synthesis and antiviral activities of N-mono- and/or N,N'-di...
2008-07-01 [Chem. Pharm. Bull. 56(7) , 1052-8, (2008)] |
|
Stereoselective Synthesis of (1R, 2S, 3R
[J. Org. Chem. 65(15) , 4753-55, (2000)] |
|
In silico fragment-based discovery of DPP-IV S1 pocket binde...
2006-03-01 [Bioorg. Med. Chem. Lett. 16 , 1405-9, (2006)] |
|
Analysis of leucine enkephalin by high-performance liquid ch...
1999-05-01 [J. Pharm. Biomed. Anal. 19(6) , 855-64, (1999)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved