Synthesis and antiviral activities of N-mono- and/or N,N'-di-carbamoyl and acyl derivatives of symmetrical diamines.
Nobuko Mibu, Kazumi Yokomizo, Marumi Oishi, Takeshi Miyata, Kunihiro Sumoto
Index: Chem. Pharm. Bull. 56(7) , 1052-8, (2008)
Full Text: HTML
Abstract
N-carbamoyl and N-acyl diamine derivatives were synthesized from symmetrical diamines by their addition to iso(thio)cyanates, cleavage reaction of acid anhydride, or N-acylation by acyl chloride. (1R,2R)-1,2-Diaminocyclohexane [(1R,2R)-1], meso-1,2-diaminocyclohexane (meso-1), (1R,2R)-1,2-diphenylethylenediamine [(1R,2R)-3], or meso-1,2-diphenylethylenediamine (meso-3) were used as the starting symmetrical diamines. The target compounds synthesized herein were evaluated for antiviral activity with herpes simplex virus type 1 (HSV-1). A few derivatives of 1,2-diaminocyclohexane [(1R,2R)-7aa and cis-7b] with adamantyl group(s) showed significant antiviral activity (EC(50)=16.0, 27.0 microg/ml).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
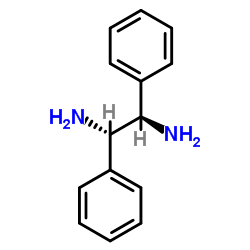 |
meso-1,2-Diphenylethylenediamine
CAS:951-87-1 |
C14H16N2 |
|
Synthesis and characterisation of nickel Schiff base complex...
2015-02-21 [Dalton Trans. 44(7) , 3136-50, (2015)] |
|
Enantioseparation of 1-arylethanols via a supramolecular chi...
2012-03-07 [Org. Biomol. Chem. 10(9) , 1877-82, (2012)] |
|
Stereoselective Synthesis of (1R, 2S, 3R
[J. Org. Chem. 65(15) , 4753-55, (2000)] |
|
In silico fragment-based discovery of DPP-IV S1 pocket binde...
2006-03-01 [Bioorg. Med. Chem. Lett. 16 , 1405-9, (2006)] |
|
Analysis of leucine enkephalin by high-performance liquid ch...
1999-05-01 [J. Pharm. Biomed. Anal. 19(6) , 855-64, (1999)] |