3-Nitropyrrole and 5-nitroindole as universal bases in primers for DNA sequencing and PCR.
D Loakes, D M Brown, S Linde, F Hill
Index: Nucleic Acids Res. 23(13) , 2361-6, (1995)
Full Text: HTML
Abstract
3-Nitropyrrole and 5-nitroindole have been assessed as universal bases in primers for dideoxy DNA sequencing and in the polymerase chain reaction (PCR). In contrast to a previous report, we have found that the introduction of more than one 3-nitropyrrole residue at dispersed positions into primers significantly reduced their efficiency in PCR and sequencing reactions. Primers containing 5-nitroindole at multiple dispersed positions were similarly affected; for both bases only a small number of substitutions were tolerated. In PCR experiments neither base, when incorporated into primers in codon third positions, was as effective as hypoxanthine, which was incorporated in six codon third positions in a 20mer oligomer. However, primers containing up to four consecutive 5-nitroindole substitutions performed well in both PCR and sequencing reactions. Consecutive 3-nitropyrrole substitutions were tolerated, but less well in comparable reactions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
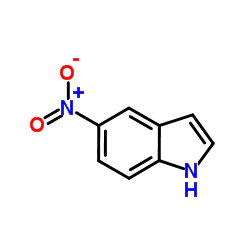 |
5-Nitroindole
CAS:6146-52-7 |
C8H6N2O2 |
|
Generic expansion of the substrate spectrum of a DNA polymer...
2004-06-01 [Nat. Biotechnol. 22(6) , 755-9, (2004)] |
|
Hybridization properties of long nucleic acid probes for det...
2010-11-01 [Nucleic Acids Res. 38(21) , e195, (2010)] |
|
Use of 5-nitroindole-2'-deoxyribose-5'-triphosphate for labe...
1998-01-01 [Nucleosides Nucleotides 17(1-3) , 555-64, (1998)] |
|
Replication of a universal nucleobase provides unique insigh...
2010-04-13 [Biochemistry 49(14) , 3009-23, (2010)] |
|
Stabilizating effect of 5-nitroindole (universal base) on DN...
1997-12-01 [J. Biomol. Struct. Dyn. 15(3) , 597-603, (1997)] |