Stabilizating effect of 5-nitroindole (universal base) on DNA duplexes immobilized on gel matrix.
A Kunitsyn, S Kochetkova, N Kolganova, E Tishchenko, B Gottikh, V Florentiev
Index: J. Biomol. Struct. Dyn. 15(3) , 597-603, (1997)
Full Text: HTML
Abstract
Effect of attachment of 1-(2-deoxy-beta-D-ribofuranosyl)-5-nitroindole (NIDR) to the ends of target sequence of oligonucleotides immobilized on gel micromatrix on stability of duplex formed by hybridization with DNA fragment was studied. It was shown that adjunction of NIDR to 5' as well as to 3' end results in increasing stability of duplexes although in the second case the extent of stabilization effect is essentially lower. Both 5' and 3' terminal NIDR exhibited no selectivity to the opposite base while the stabilizing effect depended dramatically on the nature of the adjacent base especially in the case of 5'-end-attached universal base. The neighborhood of purine bases decreased substantially the stabilizing effect of 5' terminal NIDR. In contrast with this, the stabilizing effect of 3' terminal NIDR was reduced only slightly by adjacent pyrimidine bases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
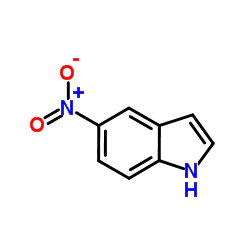 |
5-Nitroindole
CAS:6146-52-7 |
C8H6N2O2 |
|
Generic expansion of the substrate spectrum of a DNA polymer...
2004-06-01 [Nat. Biotechnol. 22(6) , 755-9, (2004)] |
|
Hybridization properties of long nucleic acid probes for det...
2010-11-01 [Nucleic Acids Res. 38(21) , e195, (2010)] |
|
Use of 5-nitroindole-2'-deoxyribose-5'-triphosphate for labe...
1998-01-01 [Nucleosides Nucleotides 17(1-3) , 555-64, (1998)] |
|
Replication of a universal nucleobase provides unique insigh...
2010-04-13 [Biochemistry 49(14) , 3009-23, (2010)] |
|
5-Nitroindole as an universal base analogue.
1994-10-11 [Nucleic Acids Res. 22(20) , 4039-43, (1994)] |