Crystallographic analysis of murine constitutive androstane receptor ligand-binding domain complexed with 5alpha-androst-16-en-3alpha-ol.
Jeremy Vincent, Li Shan, Ming Fan, Joseph S Brunzelle, Barry M Forman, Elias J Fernandez
Index: Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 61 , 156-9, (2005)
Full Text: HTML
Abstract
The constitutive androstane receptor (CAR) is a member of the nuclear receptor superfamily. In contrast to classical nuclear receptors, which possess small-molecule ligand-inducible activity, CAR exhibits constitutive transcriptional activity in the apparent absence of ligand. CAR is among the most important transcription factors; it coordinately regulates the expression of microsomal cytochrome P450 genes and other drug-metabolizing enzymes. The murine CAR ligand-binding domain (LBD) was coexpressed with the steroid receptor coactivator protein (SRC-1) receptor-interacting domain (RID) in Escherichia coli. The mCAR LBD subunit was purified away from SRC-1 by affinity, anion-exchange and size-exclusion chromatography, crystallized with androstenol and the structure of the complex determined by molecular replacement.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
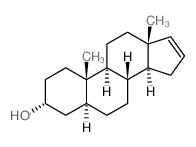 |
5alpha-Androst-16-en-3alpha-ol
CAS:1153-51-1 |
C19H30O |
|
β-cyclodextrin in personal care formulations: role on the co...
2015-08-01 [Int. J. Cosmet. Sci. 37 , 438-45, (2015)] |
|
The pheromone androstenol (5 alpha-androst-16-en-3 alpha-ol)...
2006-05-01 [J. Pharmacol. Exp. Ther. 317(2) , 694-703, (2006)] |
|
Molecular endocrinology. Two orphans find a home.
1998-10-08 [Nature 395(6702) , 543-4, (1998)] |
|
Ligand specificity of constitutive androstane receptor as pr...
2008-11-27 [J. Med. Chem. 51 , 7119-31, (2008)] |
|
Positive relationship between menstrual synchrony and abilit...
2000-08-01 [Chem. Senses 25(4) , 407-11, (2000)] |