| Structure | Name/CAS No. | Articles |
|---|---|---|
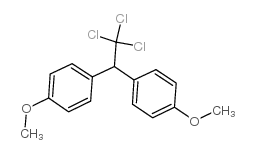 |
Benzene,1,1'-(2,2,2-trichloroethylidene)bis[4-methoxy
CAS:72-43-5 |
|
 |
Clotrimazole
CAS:23593-75-1 |
|
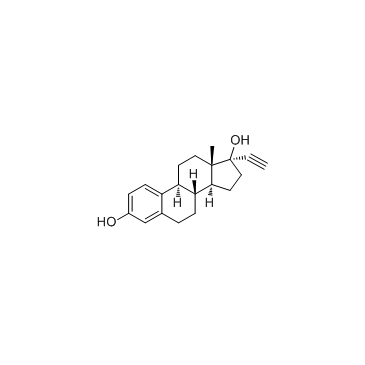 |
Ethynyl estradiol
CAS:57-63-6 |
|
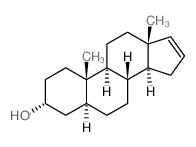 |
5alpha-Androst-16-en-3alpha-ol
CAS:1153-51-1 |