| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
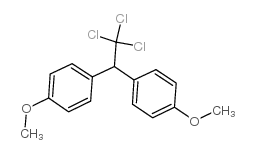 |
甲氧滴滴涕
CAS:72-43-5 |
|
 |
克霉唑
CAS:23593-75-1 |
|
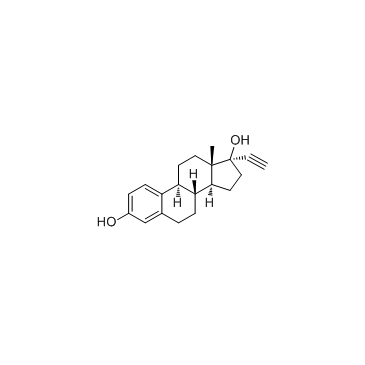 |
炔雌醇
CAS:57-63-6 |
|
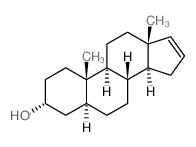 |
(3α,5α)-16-烯-3-甾醇
CAS:1153-51-1 |