Biological & Pharmaceutical Bulletin
2001-07-01
Thymidine phosphorylase inhibitors with a homophthalimide skeleton.
T Kita, H Takahashi, Y Hashimoto
Index: Biol. Pharm. Bull. 24(7) , 860-2, (2001)
Full Text: HTML
Abstract
Several N-phenylhomophthalimide derivatives were prepared and their inhibitory activity on thymidine phosphorylase/ platelet-derived endothelial cell growth factor (TP/PD-ECGF) was assessed. Among them, 2-(2,6-diethylphenyl)-7-nitro-1,2,3,4-tetrahydroisoquinoline-1,3-dione (9) was found to be a more potent inhibitor than the classical inhibitor, 5-nitrouracil (1). Lineweaver-Burk plot analysis indicated that 9 shows mixed-type competitive inhibition of TP/PD-ECGF, while 1 is a competitive inhibitor.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
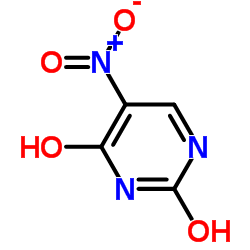 |
2,4-Dihydroxy-5-nitropyrimidine
CAS:611-08-5 |
C4H3N3O4 |
Related Articles:
More...
|
Phenobarbital induction and chemical synergism demonstrate t...
2014-12-01 [Antimicrob. Agents Chemother. 58(12) , 7475-83, (2014)] |
|
Inhibition of UDP-glucuronyltransferase activity in rat live...
1995-11-01 [Comp. Biochem. Physiol. C, Pharmacol. Toxicol. Endocrinol. 112(3) , 321-5, (1995)] |
|
The significance of 3H-thymidine degradation in cell culture...
1988-09-01 [APMIS 96(9) , 768-72, (1988)] |
|
[Conformational analysis of 5-substituted uracil].
1989-01-01 [Biofizika 34(5) , 753-7, (1989)] |
|
Catabolism of 5-fluoro-2'-deoxyuridine by isolated rat intes...
1988-12-01 [Proc. Soc. Exp. Biol. Med. 189(3) , 353-61, (1988)] |