Laser-induced photo-cross-linking of cisplatin-modified DNA to HMG-domain proteins.
Y Mikata, Q He, S J Lippard
Index: Biochemistry 40(25) , 7533-41, (2001)
Full Text: HTML
Abstract
Laser-induced photo-cross-linking was investigated for DNA, modified with cisplatin at specific sites, bound to structure-specific recognition domains of proteins in the high-mobility group (HMG) class. The efficiency of photo-cross-linking depends on the wavelength and power of the laser, the nature of the protein domain, and the oligodeoxyribonucleotide sequences flanking the platinated site. Introduction of 5-iodouridine at thymine sites of the oligodeoxyribonucleotide as an additional photoreactive group did not increase the photo-cross-linking yield. Formation of platinum-mediated DNA-DNA interstrand cross-linking observed previously upon irradiation with 302 nm light [Kane, S. A., and Lippard, S. J. (1996) Biochemistry 35, 2180-2188] was significantly reduced with laser irradiation. HMG1 domain B is superior to domain A for platinum-mediated photo-cross-linking, a result attributed to the different positioning of the proteins with respect to the platinum adduct and the greater ability of domain B to access photolabilized platinum in the major groove. Studies with proteins containing specifically mutated amino acids, and with DNA probes in which the sequences flanking the platinum cross-link site were varied, suggest that the most effective photo-cross-linking occurs for protein domains bound symmetrically and flexibly to cisplatin-modified DNA. The thermodynamic equilibrium between the protein-platinated DNA complex and its components, revealed in gel electrophoretic mobility shift assays (EMSAs), is significantly shifted to the right upon irreversible photo-cross-linking. Thus, only upon photo-cross-linking can the interaction of cisplatin-DNA 1,3-intrastrand d(GpTpG) or interstrand cross-links with HMG1 domain B protein be detected. Photo-cross-linking is thus an effective tool for investigating the interaction of cisplatin-modified DNA with damage-recognition proteins under heterogeneous conditions such those in cell extracts or living cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
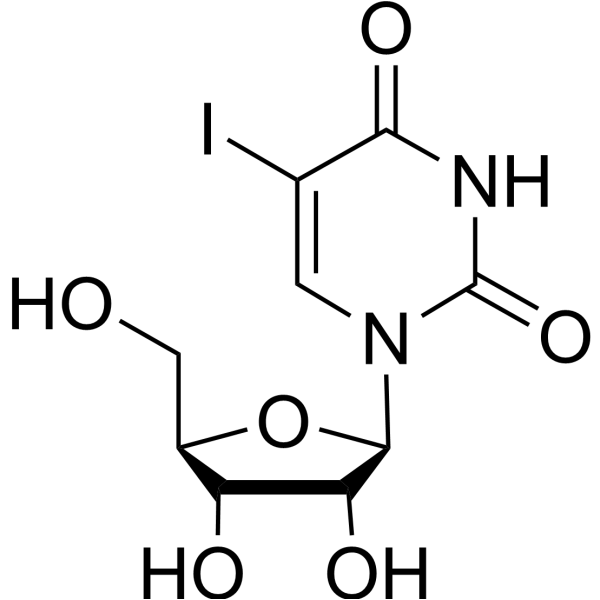 |
5-Iodouridine
CAS:1024-99-3 |
C9H11IN2O6 |
|
Loss of Foxm1 Results in Reduced Somatotrope Cell Number dur...
2015-01-01 [PLoS ONE 10 , e0128942, (2015)] |
|
Synthesis of uridine phosphoramidite analogs: reagents for s...
1994-01-01 [Bioconjug. Chem. 5(6) , 508-12, (1994)] |
|
Phosphine containing oligonucleotides for the development of...
2007-04-21 [Chem. Commun. (Camb.) (15) , 1556-8, (2007)] |
|
Crosslinking of an iodo-uridine-RNA hairpin to a single site...
1995-03-01 [RNA 1(1) , 55-63, (1995)] |
|
X-ray crystallography of large RNAs: heavy-atom derivatives ...
1996-12-01 [RNA 2(12) , 1295-305, (1996)] |