DAST-mediated regioselective anomeric group migration in saccharides.
Po-Chiao Lin, Avijit Kumar Adak, Shau-Hua Ueng, Li-De Huang, Kuo-Ting Huang, Ja-an Annie Ho, Chun-Cheng Lin
Index: J. Org. Chem. 74(11) , 4041-8, (2009)
Full Text: HTML
Abstract
When saccharides bearing a sulfur, selenium, or oxygen substituent at the anomeric center and an unprotected hydroxyl group either at C-4 or C-6 were subjected to fluorination with DAST in dichloromethane, a regioselective migration of the anomeric substituent to the C-4 or C-6 position was observed. Certain saccharides gave a mixture of migration and normal fluorination products whereas others yielded mainly or exclusively migration products (beta-glycosyl fluorides). The high thermal and chemical stability of migrated glycosyl fluorides were demonstrated to be an important precursor for many significant carbohydrate analogies. It is therefore suggested that these migrations may have useful applications in organic synthesis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
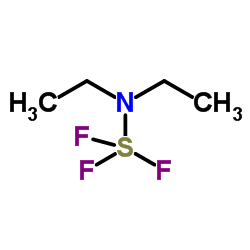 |
Diethylaminosulfur trifluoride
CAS:38078-09-0 |
C4H10F3NS |
|
Rearrangement of homoallylic alcohols induced by DAST.
2006-05-11 [Org. Lett. 8 , 2091, (2006)] |
|
Fluorinative Beckmann fragmentation: fluorinative alpha-clea...
2000-02-01 [Chem. Pharm. Bull. 48(2) , 220-2, (2000)] |
|
Synthesis of functionalized oxazolines and oxazoles with DAS...
2000-04-20 [Org. Lett. 2(8) , 1165-8, (2000)] |
|
Ring expansion of cyclic β-amino alcohols induced by diethyl...
2012-07-20 [J. Org. Chem. 77(14) , 6087-99, (2012)] |
|
Solid-phase chemical synthesis and in vitro biological evalu...
2012-11-01 [Steroids 77(13) , 1403-18, (2012)] |