[New nucleoside conjugates with phospholipids: synthesis of anti-HIV activity of rac-1-hexadecyl-2-acyl-sn-glycero-3-phosphonucleosides].
S I Malekin, Iu L Krugliak, N Iu Khromova, M Iu Aksenova, V P Sokolov, A V Kisin, T I Novozhilova, S G Popova, V K Kurochkin, G G Miller, L N Pokidysheva
Index: Bioorg. Khim. 23(8) , 648-54, (1997)
Full Text: HTML
Abstract
New nucleoside-phospholipid conjugates were synthesized based on 1,2-disubstituted glycerides and nucleosides. These contain rac-1-hexadecyl-2-palmitoyl(or 2-methylcarbamoyl)-sn-glycero-3-phosphate as the phospholipid component and 2',3'-didehydro-3'-deoxythymidine, 1-(Z-5-hydroxypentene-2-yl)thymine, or 2',3'-isopropylideneuridine as a nucleoside component. The conjugates were synthesized by three different ways: from rac-1-hexadecyl-2-acyl-sn-glycero-3-phospodichlorides, -3-phosphatidic acids, or -3-H-phosphonates. When subjected to mild alkaline hydrolysis, conjugates containing a 2-palmitoyl group formed conjugates with the lysophospholipid component that had not yet been described. All the conjugates obtained were amorphous compounds stable at room temperature. Their hemolytic and anti-HIV activities were determined. Some conjugates were found to completely inhibit in vitro HIV-1 reproduction in lower doses than the corresponding nucleosides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
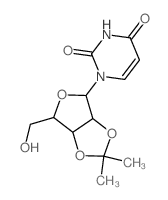 |
2',3'-o-isopropylideneuridine
CAS:362-43-6 |
C12H16N2O6 |
|
5'-Uridyl derivatives of N-glycosyl allophanic acid and biur...
2010-01-11 [Carbohydr. Res. 345 , 163-167, (2010)] |
|
New methods for the synthesis of N-benzoylated uridine and t...
2002-02-18 [Carbohydr. Res. 337 , 369-372, (2002)] |
|
Synthesis and central nervous system depressant effects of N...
1999-12-01 [Chem. Pharm. Bull. 47 , 1802-1804, (1999)] |
|
Synthesis of N3-substituted uridine and related pyrimidine n...
2005-03-01 [Chem. Pharm. Bull. 53 , 313-318, (2005)] |
|
Enhancement of radiation-induced base release from nucleosid...
1992-04-01 [Int. J. Radiat. Biol. 61(4) , 443-9, (1992)] |