Synthesis of N3-substituted uridine and related pyrimidine nucleosides and their antinociceptive effects in mice.
Tomomi Shimizu, Toshiyuki Kimura, Tatsuya Funahashi, Kazuhito Watanabe, Ing Kang Ho, Ikuo Yamamoto
Index: Chem. Pharm. Bull. 53 , 313-318, (2005)
Full Text: HTML
Abstract
Seventy eight N(3)-substituted derivatives of uridine (1), thymidine (2), 2'-deoxyuridine (3), 6-azauridine (4), 2',3'-O-isopropylideneuridine (5), and arabinofuranosyluracil (6) were synthesized and their antinociceptive effects were evaluated. N(3)-(2',4'-Dimethoxyphenacyl)uridine (1l), N(3)-(2',4'-dimethoxyphenacyl)2'-deoxyuridine (3l), and N(3)-(2',5'-dimethoxyphenacyl)arabinofuranosyluracil (6m) possessed 93, 86, and 82% of the antinociceptive effects tested by hot plate, respectively. The antinociceptive effects of three derivatives were 5.8, 5.4, and 5.1-folds of the effect of N(3)-phenacyluridine (1h) (16%), respectively. The structure-activity relationship of N(3)-substituted pyrimidine nucleosides was also discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
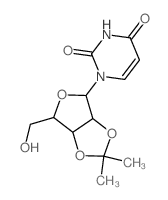 |
2',3'-o-isopropylideneuridine
CAS:362-43-6 |
C12H16N2O6 |
|
5'-Uridyl derivatives of N-glycosyl allophanic acid and biur...
2010-01-11 [Carbohydr. Res. 345 , 163-167, (2010)] |
|
New methods for the synthesis of N-benzoylated uridine and t...
2002-02-18 [Carbohydr. Res. 337 , 369-372, (2002)] |
|
Synthesis and central nervous system depressant effects of N...
1999-12-01 [Chem. Pharm. Bull. 47 , 1802-1804, (1999)] |
|
Enhancement of radiation-induced base release from nucleosid...
1992-04-01 [Int. J. Radiat. Biol. 61(4) , 443-9, (1992)] |
|
[New nucleoside conjugates with phospholipids: synthesis of ...
1997-08-01 [Bioorg. Khim. 23(8) , 648-54, (1997)] |