| Structure | Name/CAS No. | Articles |
|---|---|---|
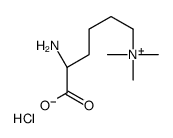 |
Nε,Nε,Nε-Trimethyllysine chloride
CAS:55528-53-5 |
|
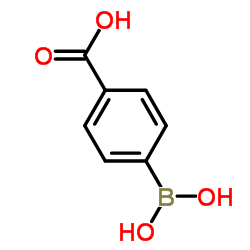 |
p-Boronobenzoic acid
CAS:14047-29-1 |
|
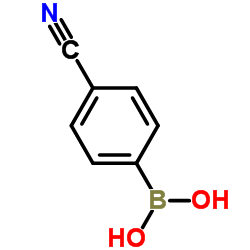 |
4-Cyanophenylboronic acid
CAS:126747-14-6 |