Kinetic manifestation of processivity during multiple methylations catalyzed by SET domain protein methyltransferases.
Lynnette M A Dirk, E Megan Flynn, Kevin Dietzel, Jean-François Couture, Raymond C Trievel, Robert L Houtz
Index: Biochemistry 46(12) , 3905-15, (2007)
Full Text: HTML
Abstract
Processive versus distributive methyl group transfer was assessed for pea Rubisco large subunit methyltransferase, a SET domain protein lysine methyltransferase catalyzing the formation of trimethyllysine-14 in the large subunit of Rubisco. Catalytically competent complexes between an immobilized form of des(methyl) Rubisco and Rubisco large subunit methyltransferase were used to demonstrate enzyme release that was co-incident with and dependent on formation of trimethyllysine. Catalytic rate constants determined for formation of trimethyllysine were considerably lower ( approximately 10-fold) than rate constants determined for total radiolabel incorporation from [3H-methyl]-S-adenosylmethionine. Double-reciprocal velocity plots under catalytic conditions favoring monomethyllysine indicated a random or ordered reaction mechanism, while conditions favoring trimethyllysine suggested a hybrid ping-pong mechanism. These results were compared with double-reciprocal velocity plots and product analyses obtained for HsSET7/9 (a monomethyltransferase) and SpCLR4 (a dimethyltransferase) and suggest a predictive ability of double-reciprocal velocity plots for single versus multiple methyl group transfers by SET domain protein lysine methyltransferases. A model is proposed for SET domain protein lysine methyltransferases in which initial binding of polypeptide substrate and S-adenosylmethionine is random, with polypeptide binding followed by deprotonation of the epsilon-amine of the target lysyl residue and subsequent methylation. Following methyl group transfer, S-adenosylhomocysteine and monomethylated polypeptide dissociate from monomethyltransferases, but di- and trimethyltransferases begin a successive and catalytically obligatory deprotonation of enzyme-bound methylated lysyl intermediates, which along with binding and release of S-adenosylmethionine and S-adenosylhomocysteine is manifested as a hybrid ping-pong-like reaction mechanism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
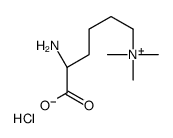 |
Nε,Nε,Nε-Trimethyllysine chloride
CAS:55528-53-5 |
C9H21ClN2O2 |
|
Inhibition of histone binding by supramolecular hosts.
2014-05-01 [Biochem. J. 459(3) , 505-12, (2014)] |
|
A new type of protein lysine methyltransferase trimethylates...
2014-12-12 [Biochem. Biophys. Res. Commun. 455(3-4) , 382-9, (2014)] |
|
Tuning HP1α chromodomain selectivity for di- and trimethylly...
2011-12-16 [ChemBioChem. 12(18) , 2786-90, (2011)] |
|
Biosynthesis of proteins containing modified lysines and flu...
2011-04-01 [J. Biosci. Bioeng. 111(4) , 402-7, (2011)] |
|
Treatment with pharmacological peroxisome proliferator-activ...
2007-08-01 [Pharmacol. Res. 56(2) , 175-83, (2007)] |