Naphthylphenstatins as tubulin ligands: synthesis and biological evaluation.
Concepción Alvarez, Raquel Alvarez, Purificación Corchete, Concepción Pérez-Melero, Rafael Peláez, Manuel Medarde
Index: Bioorg. Med. Chem. 16(19) , 8999-9008, (2008)
Full Text: HTML
Abstract
A new family of naphthalenic analogues of phenstatins with modifications on the ketone-bridge has been synthesised. The synthesised compounds have been assayed for tubulin polymerisation inhibitory activity as well as for cytotoxic activity against cancer cell lines. The naphthalene has been confirmed as a good surrogate for the isovanillin moiety (3-hydroxy-4-methoxyphenyl) of phenstatin, when combined with the 3,4,5-trimethoxyphenyl ring, but not when combines with the 2,3,4-trimethoxyphenyl ring. Binding models for the synthesised compounds have been generated and analysed in terms of a pharmacophore proposed for colchicine site ligands. The ketone is the optimal bridge substitution but E-acetyloximes or acetylhydrazones are also tolerated. Significant differences with indole substituted phenstatins are observed and discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
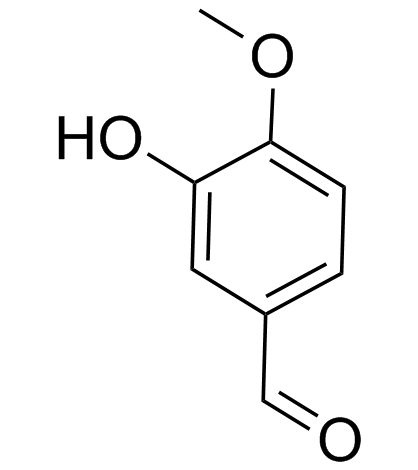 |
Isovanillin
CAS:621-59-0 |
C8H8O3 |
|
Synthesis and accumulation of aromatic aldehydes in an engin...
2014-08-20 [J. Am. Chem. Soc. 136(33) , 11644-54, (2014)] |
|
3D-QSAR and molecular docking studies of benzaldehyde thiose...
2007-03-01 [Bioorg. Med. Chem. 15 , 2006-15, (2007)] |
|
Systemic exposure to and disposition of catechols derived fr...
2015-05-01 [Drug Metab. Dispos. 43(5) , 679-90, (2015)] |
|
Pd(II)-mediated cyclization of o-allylbenzaldehydes in water...
2012-09-21 [Org. Lett. 14(18) , 4930-3, (2012)] |
|
Aldehyde oxidase: an enzyme of emerging importance in drug d...
2010-12-23 [J. Med. Chem. 53 , 8441-60, (2010)] |