| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Palladium chloride
CAS:7647-10-1 |
|
 |
Cupric chloride
CAS:7447-39-4 |
|
 |
Copper(II) chloride dihydrate
CAS:10125-13-0 |
|
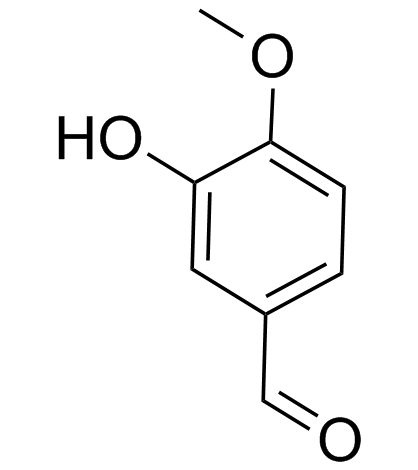 |
Isovanillin
CAS:621-59-0 |