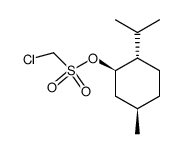
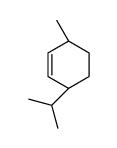
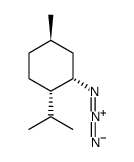
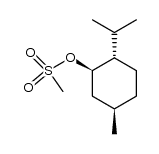
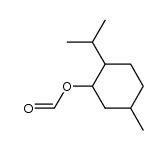
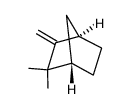
|
~% |
|
~96% |
|
~% |
|
~% |
|
~%
Detail
|
|
~92% |
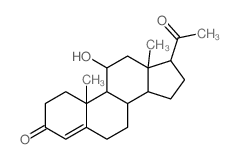
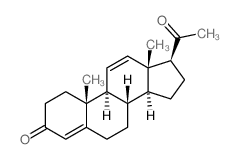
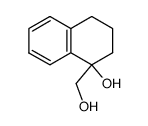
![8,9-Dihydro-5H-benzo[7]annulen-6(7H)-one Structure](https://image.chemsrc.com/caspic/155/34663-15-5.png)