Metabolic effects of propranolol and hydroflumethiazide treatment in Kenyans with mild to moderate essential hypertension.
G O Yonga, E N Ogola, D A Orinda
Index: East Afr. Med. J. 70(11) , 696-700, (1993)
Full Text: HTML
Abstract
In a prospective single-blind comparative trial, sixty newly diagnosed mild to moderate hypertensives were randomly assigned to either propranolol or hydroflumethiazide monotherapy. Baseline fasting serum glucose lipid profiles, serum uric acid and potassium levels, were determined at the beginning of the trial. Repeat levels were determined at completion of twelve weeks of treatment. Propranolol treatment significantly reduced HDL-cholesterol (p < 0.02) and increased both VLDL and total serum triglycerides (p < 0.01). Hydroflumethiazide significantly increased total and LDL-chole-sterol, fasting serum glucose and uric acid levels (p < 0.01); potassium levels were significantly lowered (p < 0.01). Treatment with either propranolol or hydroflumethiazide is associated with significant metabolic side-effects which require regular monitoring and intervention as appropriate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
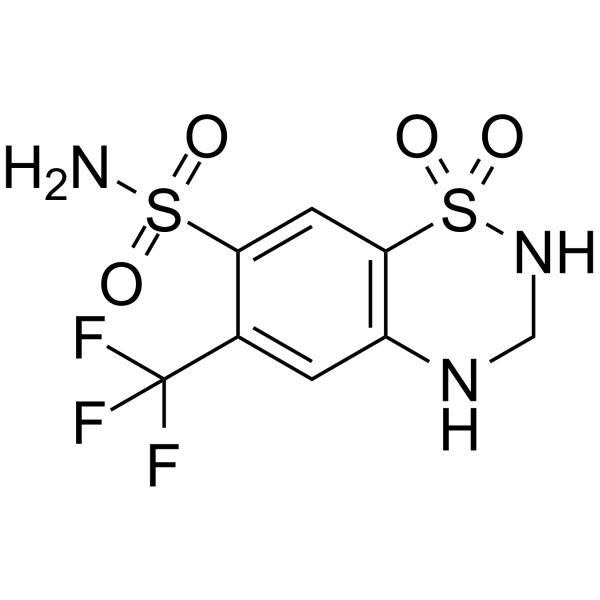 |
hydroflumethiazide
CAS:135-09-1 |
C8H8F3N3O4S2 |
|
Do thiazides reduce intestinal oxalate absorption?: a study ...
1994-03-01 [Clin. Sci. 86(3) , 353-7, (1994)] |
|
Comparative effects of LN 5330 and diazoxide on insulin rele...
1991-07-25 [Biochem. Pharmacol. 42(4) , 787-91, (1991)] |
|
Photoaugmentation effects demonstrated in vitro.
1996-12-01 [Photodermatol. Photoimmunol. Photomed. 12(6) , 227-32, (1996)] |
|
Phototoxicity to sulphonamide-derived oral antidiabetics and...
1997-01-01 [Photodermatol. Photoimmunol. Photomed. 13(1-2) , 4-8, (1997)] |
|
Rapid determination of losartan and losartan acid in human p...
2009-10-01 [J. Sep. Sci. 32(20) , 3388-94, (2009)] |