Eudragit E as excipient for production of granules and tablets from Phyllanthus niruri L spray-dried extract.
Tatiane Pereira de Souza, Ramón Martínez-Pacheco, José Luiz Gómez-Amoza, Pedro Ros Petrovick
Index: AAPS PharmSciTech 8(2) , Article 34, (2007)
Full Text: HTML
Abstract
The aim of this study was to investigate the feasibility of using Eudragit E as a granulating agent for a spray-dried extract from Phyllanthus niruri to obtain tablets containing a high dose of this product. The granules were developed by wet granulation and contained 2.5%, 5.0%, and 10.0% Eudragit E in the final product concentration. The tablets were produced on a single-punch tablet press by direct compression of granules using 0.5% magnesium stearate as a lubricant. The tablets were elaborated following a 2 x 3 factorial design, where Eudragit E concentration and compression force were the independent variables, and tensile strength and the extract release of the tablets were the dependent variables. All granules showed better technological properties than the spray-dried extract, including less moisture sorption. The characteristics of the granules were directly dependent on the proportion of Eudragit E in the formulation. In general, all tablets showed high mechanical resistance with less than 1% friability, less moisture sorption, and a slower extract release profile. The Eudragit E concentration and compression force of the tablets significantly influenced both dependent variables studied. In conclusion, Eudragit E was efficient as a granulating agent for the spray-dried extract, but additional studies are needed to further optimize the formulations in order to achieve less water sorption and improve the release of the extract from the tablets.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
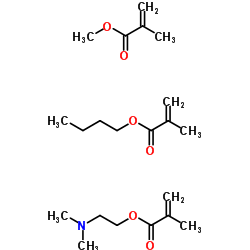 |
Amino methacrylate copolymer
CAS:24938-16-7 |
C21H37NO6 |
|
Improved supersaturation and oral absorption of dutasteride ...
2012-01-01 [Chem. Pharm. Bull. 60(11) , 1468-73, (2012)] |
|
Effect of aminoalkyl methacrylate copolymer E/HCl on in vivo...
2013-11-01 [Drug Dev. Ind. Pharm. 39(11) , 1698-705, (2013)] |
|
Studies on gynaecological hydrophilic lactic acid preparatio...
2003-04-01 [Pharmazie 58(4) , 260-2, (2003)] |
|
Evaluation of in vitro dissolution and in vivo oral absorpti...
2013-06-01 [Drug Res. (Stuttg.) 63(6) , 326-30, (2013)] |
|
In vitro evaluation of dissolution behavior for a colon-spec...
2002-01-01 [AAPS PharmSciTech 3(4) , E33, (2002)] |