Effect of aminoalkyl methacrylate copolymer E/HCl on in vivo absorption of poorly water-soluble drug.
Takatsune Yoshida, Ippei Kurimoto, Keiichi Yoshihara, Hiroyuki Umejima, Naoki Ito, Shunsuke Watanabe, Kazuhiro Sako, Akihiko Kikuchi
Index: Drug Dev. Ind. Pharm. 39(11) , 1698-705, (2013)
Full Text: HTML
Abstract
This study aimed to investigate in vivo absorption of tacrolimus formulated as a solid dispersion using Eudragit E®/HCl (E-SD). E-SD is an aminoalkyl methacrylate copolymer that can be dissolved under neutral pH conditions. E-SD was used alone as a solid dispersion carrier and/or was mixed with tacrolimus primarily dispersed with hydroxypropylmethylcellulose (HPMC). Tacrolimus was formulated with E-SD at several different ratios. Formulations with tacrolimus/E-SD ratio of 1/3 showed higher in vivo absorption, compared to tacrolimus dispersed in the excipients (primarily HPMC) found in commercially available tacrolimus capsules, using a rat in situ closed loop method. Good correlation was observed between in vitro drug solubility and in vivo drug absorption. In vitro solubility tests and rat oral absorption studies of tacrolimus/HPMC solid dispersion formulations were also conducted after mixing the HPMC dispersion with several ratios of E-SD. E-SD/tacrolimus/HPMC formulations yielded high in vitro drug solubility but comparatively low in vivo absorption. Dog oral absorption studies were conducted using capsules containing a formulation of tacrolimus/E-SD at a ratio of 1/5. The E-SD formulation-containing capsule showed higher in vivo drug absorption than tacrolimus dispersed in the standard HPMC capsule. These studies report enhancement of the in vivo absorption of a poorly water-soluble drug following dispersion with E-SD when compared to formulation in HPMC.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
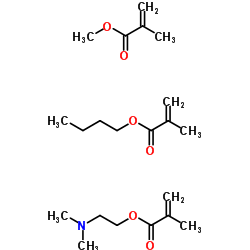 |
Amino methacrylate copolymer
CAS:24938-16-7 |
C21H37NO6 |
|
Improved supersaturation and oral absorption of dutasteride ...
2012-01-01 [Chem. Pharm. Bull. 60(11) , 1468-73, (2012)] |
|
Eudragit E as excipient for production of granules and table...
2007-01-01 [AAPS PharmSciTech 8(2) , Article 34, (2007)] |
|
Studies on gynaecological hydrophilic lactic acid preparatio...
2003-04-01 [Pharmazie 58(4) , 260-2, (2003)] |
|
Evaluation of in vitro dissolution and in vivo oral absorpti...
2013-06-01 [Drug Res. (Stuttg.) 63(6) , 326-30, (2013)] |
|
In vitro evaluation of dissolution behavior for a colon-spec...
2002-01-01 [AAPS PharmSciTech 3(4) , E33, (2002)] |