Mannose 6-phosphate receptor homology (MRH) domain-containing lectins in the secretory pathway.
Alicia C Castonguay, Linda J Olson, Nancy M Dahms
Index: Biochim. Biophys. Acta 1810(9) , 815-26, (2011)
Full Text: HTML
Abstract
The mannose 6-phosphate receptor homology (MRH) domain-containing family of proteins, which include recycling receptors (mannose 6-phosphate receptors, MPRs), resident endoplasmic reticulum (ER) proteins (glucosidase II β-subunit, XTP3-B, OS-9), and a Golgi glycosyltransferase (GlcNAc-phosphotransferase γ-subunit), are characterized by the presence of one or more MRH domains. Many MRH domains act as lectins and bind specific phosphorylated (MPRs) or non-phosphorylated (glucosidase II β-subunit, XTP3-B and OS-9) high mannose-type N-glycans. The MPRs are the only proteins known to bind mannose 6-phosphate (Man-6-P) residues via their MRH domains.Recent biochemical and structural studies that have provided valuable insight into the glycan specificity and mechanisms of carbohydrate recognition by this diverse group of MRH domain-containing proteins are highlighted.Currently, three-dimensional structures are known for ten MRH domains, revealing the conservation of a similar fold. OS-9 and the MPRs use the same four residues (Gln, Arg, Glu, and Tyr) to bind mannose.The MRH domain-containing proteins play key roles in the secretory pathway: glucosidase II, XTP3-B, and OS-9 are involved in the recognition of nascent glycoproteins, whereas the MPRs play an essential role in lysosome biogenesis by targeting Man-6-P-containing lysosomal enzymes to the lysosome.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
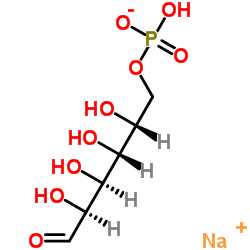 |
Sodium (2R,3R,4S,5S)-2,3,4,5-tetrahydroxy-6-oxohexyl hydrogenphosphate
CAS:70442-25-0 |
C6H12NaO9P |
|
Biomineralization in diatoms-phosphorylated saccharides are ...
2013-01-10 [Carbohydr. Res. 365 , 52-60, (2013)] |
|
The DMAP interaction domain of UDP-GlcNAc:lysosomal enzyme N...
2013-06-18 [Proc. Natl. Acad. Sci. U. S. A. 110(25) , 10246-51, (2013)] |
|
Mannose-6-phosphate regulates destruction of lipid-linked ol...
2011-09-01 [Mol. Biol. Cell 22(17) , 2994-3009, (2011)] |
|
In vivo targeting of alveolar macrophages via RAFT-based gly...
2012-10-01 [Biomaterials , (2012)] |
|
Mannose 6 phosphorylation of lysosomal enzymes controls B ce...
2015-01-19 [J. Cell Biol. 208(2) , 171-80, (2015)] |