| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Endoproteinase Arg-C
CAS:82047-85-6 |
|
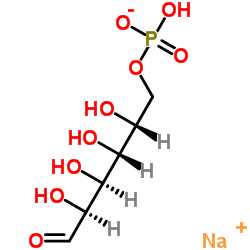 |
Sodium (2R,3R,4S,5S)-2,3,4,5-tetrahydroxy-6-oxohexyl hydrogenphosphate
CAS:70442-25-0 |