Zeitschrift fuer Naturforschung. Section C
2008-01-01
Ketoisophorone transformation by Marchantia polymorpha and Nicotiana tabacum cultured cells.
Mohamed-Elamir F Hegazy, Toshifumi Hirata, Ahmed Abdel-Lateff, Mohamed H Abd el-Razek, Abou El-Hamd H Mohamed, Nahed M Hassan, Paul W Paré, Ahmed A Mahmoud
Index: Z. Naturforsch., C, J. Biosci. 63(5-6) , 403-8, (2008)
Full Text: HTML
Abstract
Stereospecific olefin (C=C) and carbonyl (C=O) reduction of the readily available prochiral compound ketoisophorone (2,2,6-trimethyl-2-cyclohexene-1,4-dione) (1) by Marchantia polymorpha and Nicotiana tabacum cell suspension cultures produce the chiral products (6R)-levodione (2), (4R,5S)-4-hydroxy-3,3,5-trimethylcyclohexanone (3), and (4R,6R)-actinol (4) as well as the minor components (4R)-hydroxyisophorone (5) and (4S)-phorenol (6).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
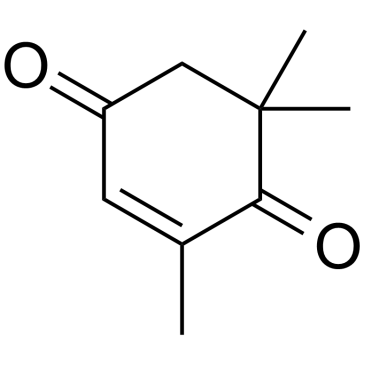 |
2,6,6-Trimethyl-2-cyclohexene-1,4-dione
CAS:1125-21-9 |
C9H12O2 |
Related Articles:
More...
|
Engineering towards nitroreductase functionality in ene-redu...
2015-03-23 [ChemBioChem. 16(5) , 811-8, (2015)] |
|
Bioreduction of α,β-unsaturated ketones and aldehydes by non...
2014-12-01 [Bioresour. Technol. 102(5) , 3993-8, (2011)] |
|
An engineered old yellow enzyme that enables efficient synth...
2015-02-09 [ChemBioChem. 16(3) , 440-5, (2015)] |
|
Old Yellow Enzyme from Candida macedoniensis catalyzes the s...
2002-12-01 [Biosci. Biotechnol. Biochem. 66(12) , 2651-7, (2002)] |
|
Quality and functionality of saffron: quality control, speci...
2008-06-01 [Planta Med. 74(7) , 764-72, (2008)] |