Enolates in 3-D: an experimental and computational study of deprotonated 2-adamantanone.
Matthew M Meyer, Steven R Kass
Index: J. Org. Chem. 75(12) , 4274-9, (2010)
Full Text: HTML
Abstract
Deprotonation of 2-adamantanone (1) in the gas phase affords the corresponding beta-enolate anion. This ion was independently prepared by the fluoride-induced desilylation of 4-trimethylsilyl-2-adamantanone, and its reactivity and thermodynamic properties were measured (DeltaH degrees(acid) = 394.7 +/- 1.4, EA = 16.8 +/- 1.6, and BDE = 97.9 +/- 2.1 kcal mol(-1)). Density functional theory calculations with B3LYP and M06-2X, and G3 energies are also reported. The computed relative stabilities of the conjugate bases of 1 are as follows: beta > gamma > alpha > delta. An attempt to prepare the gamma-anion, however, resulted in the formation of its ring-opened isomer (i.e., deprotonated 7-methylenebicyclo[3.3.1]nonan-2-one).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
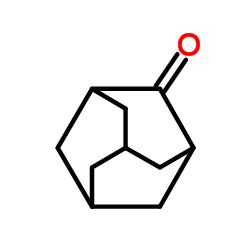 |
adamantanone
CAS:700-58-3 |
C10H14O |
|
Effect of adamantanone derivative possessing anti-HIV proper...
2001-01-01 [Dokl. Biochem. Biophys. 378 , 210-3, (2001)] |
|
Conformational relaxation in hemoproteins: the cytochrome P-...
2000-11-21 [Biochemistry 39(46) , 14219-31, (2000)] |
|
The structural basis for substrate-induced changes in redox ...
1989-01-24 [Biochemistry 28(2) , 917-22, (1989)] |
|
(+/-)-1-(Adamantan-2-yl)-2-propanamine and other amines deri...
1980-05-01 [Farmaco. Sci. 35(5) , 430-40, (1980)] |
|
Synthesis of a silene from 1,1-dilithiosilole and 2-adamanta...
2004-05-05 [J. Am. Chem. Soc. 126(17) , 5336-7, (2004)] |