Piperidine dispiro-1,2,4-trioxane analogues.
Sunil Sabbani, Paul A Stocks, Gemma L Ellis, Jill Davies, Erik Hedenstrom, Stephen A Ward, Paul M O'Neill
Index: Bioorg. Med. Chem. Lett. 18(21) , 5804-8, (2008)
Full Text: HTML
Abstract
Dispiro N-Boc-protected 1,2,4-trioxane 2 was synthesised via Mo(acac)(2) catalysed perhydrolysis of N-Boc spirooxirane followed by condensation of the resulting beta-hydroperoxy alcohol 10 with 2-adamantanone. N-Boc 1,2,4-trioxane 2 was converted to the amine 1,2,4-trioxane hydrochloride salt 3 which was subsequently used to prepare derivatives (4-7). Several of these novel 1,2,4-trioxanes had nanomolar antimalarial activity versus the 3D7 strain of Plasmodium falciparum. Amine intermediate 3 represents a versatile derivative for the preparation of achiral arrays of trioxane analogues with antimalarial activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
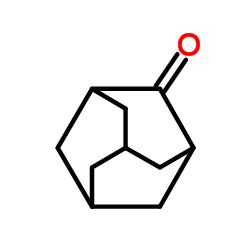 |
adamantanone
CAS:700-58-3 |
C10H14O |
|
Effect of adamantanone derivative possessing anti-HIV proper...
2001-01-01 [Dokl. Biochem. Biophys. 378 , 210-3, (2001)] |
|
Conformational relaxation in hemoproteins: the cytochrome P-...
2000-11-21 [Biochemistry 39(46) , 14219-31, (2000)] |
|
The structural basis for substrate-induced changes in redox ...
1989-01-24 [Biochemistry 28(2) , 917-22, (1989)] |
|
(+/-)-1-(Adamantan-2-yl)-2-propanamine and other amines deri...
1980-05-01 [Farmaco. Sci. 35(5) , 430-40, (1980)] |
|
Synthesis of a silene from 1,1-dilithiosilole and 2-adamanta...
2004-05-05 [J. Am. Chem. Soc. 126(17) , 5336-7, (2004)] |