A stability-indicating high-performance liquid chromatographic method for the determination of methocarbamol in veterinary preparations.
María A Rosasco, Rita Ceresole, Clara C Forastieri, Adriana I Segall
Index: J. AOAC Int. 92(5) , 1602-5, (2009)
Full Text: HTML
Abstract
An isocratic HPLC method was developed and validated for the quantitation of methocarbamol in the presence of its degradation products. Quantitation was achieved using a reversed-phase C18 column at ambient temperature with mobile phase consisting of methanol-water-tetrahydrofuran (25 + 65 + 10, v/v). The flow rate was 0.9 mL/min. The detection was by UV light at 274 nm. The proposed method was validated for selectivity, precision, linearity, and accuracy. The assay method was found to be linear from 159.0 to 793.2 microg/mL (3.2 to 15.9 microg injected). All validation parameters were within the acceptable range. The developed method was successfully applied to estimate the amount of methocarbamol in a veterinary injection.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
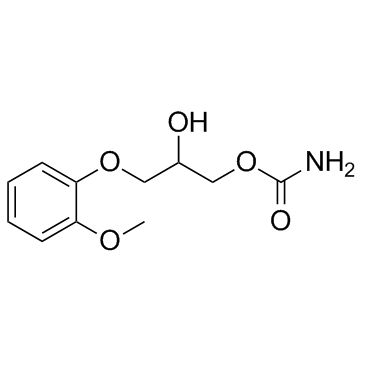 |
methocarbamol
CAS:532-03-6 |
C11H15NO5 |
|
A new HPLC technique for the separation of methocarbamol ena...
1999-07-01 [J. Pharm. Pharmacol. 51(7) , 873-5, (1999)] |
|
Spectrofluorometric determination of methocarbamol in pharma...
2011-03-01 [J. Fluoresc. 21(2) , 555-61, (2011)] |
|
Efficacy and safety of sodium hyaluronate in the treatment o...
2008-11-01 [J. Oral Maxillofac. Surg. 66(11) , 2243-6, (2008)] |
|
Simultaneous spectrophotometric determination of diclofenac ...
2011-04-01 [Drug Test. Anal. 3(4) , 228-33, (2011)] |
|
Effect of preoperative intravenous methocarbamol and intrave...
2013-02-01 [Orthopedics 36(2 Suppl) , 25-32, (2013)] |